Respiratory syncytial virus (RSV) is the leading cause of acute bronchiolitis, requiring hospitalization up to 20.3% of cases under 6 months old globally [1]. Key risk factors for severe bronchiolitis include age under 10 weeks, prematurity, chronic lung disease and congenital heart disease [2]. Regarding its prevention, passive immunization with palivizumab significantly reduced RSV incidence in at-risk populations during peak RSV season [3]. In 2022, nirsevimab was approved by EMA (European Medicines Agency) for passive immunization as well. By the 2023–2024 winter season (October 1st–March 31st), it was administered as prophylaxis to every infant under 6 months (born from April 1st, 2023) and to children under 2 years with risk factors in Spain [4]. It was government-funded and no signed informed consent was needed. A single dose of nirsevimab has been shown to reduce RSV hospitalization risk by up to 90% [5–8]. Despite the fact that palivizumab did not demonstrate therapeutic effectiveness for RSV bronchiolitis [9], no data is available on nirsevimab role when administered to non-immunized infants hospitalized for acute RSV bronchiolitis. We aimed to describe the epidemiological and clinical features of infants under 6 months admitted to acute bronchiolitis in the first season of nirsevimab. Our secondary objectives were to assess the reduction in hospitalization of RSV-positive bronchiolitis by comparing it with data from the previous season, and to elucidate if there were clinical differences between immunized and non-immunized RSV-positive cases.
This retrospective observational study reviewed the electronic medical records of infants under 6 months, with and without risk factors, hospitalized with acute bronchiolitis (ICD-10 J21.9) across three hospitals in Madrid (Spain) between October 1st, 2023, and March 31st, 2024. Demographic data, medical conditions, nirsevimab status, microbiological results and clinical outcomes were collected. We compared hospitalization for acute bronchiolitis and RSV-positivity rate with the previous season (2022–2023). Two of the hospitals are important teaching reference hospitals of Madrid with Pediatric Intensive Care Unit (PICU) with similar admission criteria and characteristics. PICU admission criteria include the need for positive-pressure oxygen therapy (CPAP or BiPAP) with nasogastric feeding associated. The third hospital has no PICU and refers patients to other hospitals if they need it. Ethical approval was granted by the Ethics Committee of each participating hospital (references: A1557, PI-6031 and R-0022/24). The study was performed in line with the Declaration of Helsinki and Spanish biomedical research legislation.
One of the two reference hospitals administered nirsevimab within the first 48h of hospitalization to all admitted infants under 6 months without it, regardless of the reason for admission to ensure passive immunization against both types of RSV, A and B. The other two hospitals administered nirsevimab at the moment of discharge to ensure complete immunization as well. In all cases, it was discussed with the parents and they gave their verbal consent. Statistical analysis was performed using SPSS version 29, with statistical significance defined as a p-value <0.05. After processing data, a post hoc analysis was conducted. A comparison between RSV-positive patients with early nirsevimab administration and without nirsevimab was conducted. Secondary, all significant variables were entered into a binary logistic regression model.
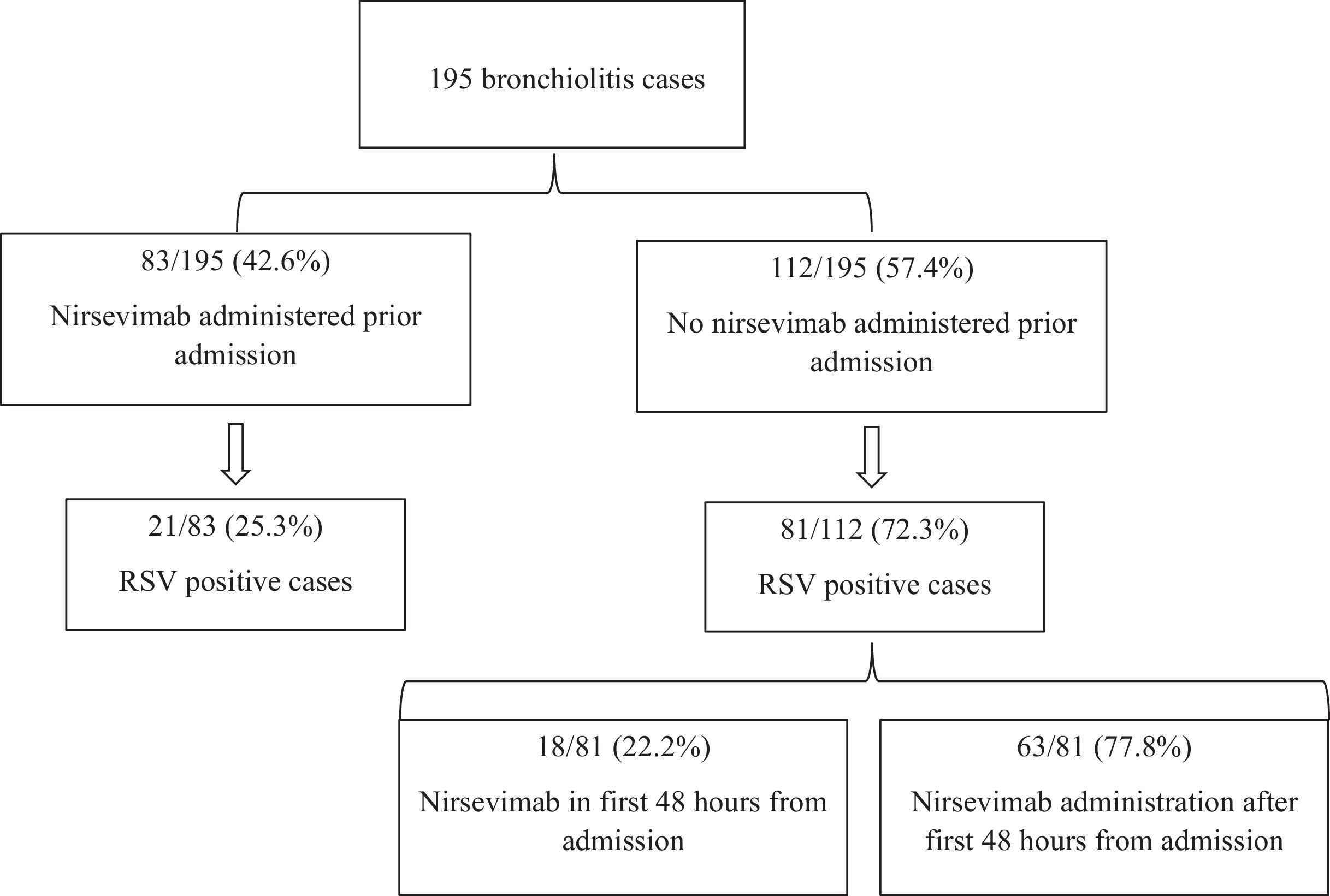
Between October 1st, 2023, and March 31st, 2024, 195 cases of acute bronchiolitis were admitted (median age 2.37 months [interquartile range, IQR, 1.17–3.87], 66.7% male). RSV was detected in 102 cases (52.5%). Respiratory support was needed in 96.4% of cases, PICU admission was required in 20.5% cases and nasogastric feeding, in 38.5% cases (characteristics of the cohort in Suppl. Table 1). Nasogastric feeding data was available only from the two reference hospitals (the third one did not prescribe it). Of all cases, 42.6% had received nirsevimab before admission (Fig. 1). Among them, 25.3% were RSV-positive, while 72.3% of non-immunized cases were RSV-positive. No differences in risk factors and clinical outcomes were observed by RSV-positive cases immunized before admission and non-immunized (Suppl. Table 2). Compared with the previous season (October 2022–March 2023) in the three hospitals, there has been a 64.5% reduction in bronchiolitis hospitalizations (550 vs. 195, p<0.001) and a 71.2% reduction in RSV bronchiolitis (354 vs. 102, p<0.001).
Distribution of cases by nirsevimab status prior to admission and RSV-positivity rate. It can be seen that 42.6% of cases had received nirsevimab before admission (25.3% of them were RSV-positive), while 57.4% of cases had not received nirsevimab prior admission (72.3% of them were RSV-positive). Among this group of non-immunized cases, 22.2% received nirsevimab within first 48h of admission.
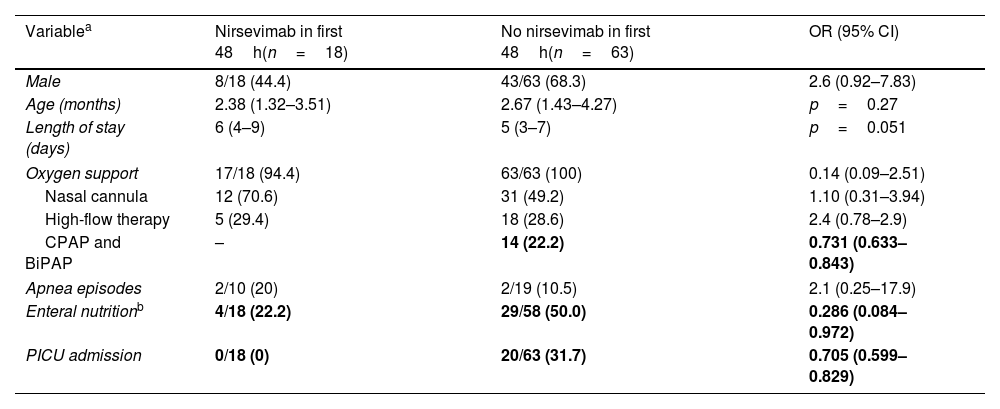
Among the 81 non-immunized RSV-positive cases, 18 received nirsevimab within the first 48h of admission (Fig. 1). After post hoc analysis, no patient with early administration required positive-pressure oxygen (CPAP or BiPAP), while 22.2% of non-early immunized patients needed positive-pressure oxygen (OR 0.73, 95% CI 0.63–0.84, Table 1). Besides, no patient with early administration was admitted to PICU, compared to 31.7% of the non-early immunized infants (OR 0.71, 95% CI 0.60–0.83, Table 1). Regarding nasogastric feeding, 22.2% of cases with early nirsevimab needed nasogastric feeding compared to 50% of cases without early nirsevimab (OR 0.29, 95% CI 0.10–0.97, Table 1). After carrying out the binary logistic regression model, it was found that children who did not receive nirsevimab in the first 48h needed more nasogastric feeding (OR 4.03, 95% CI 1.17–13.85). Sex and age were not significantly different between groups. Positive-pressure oxygen and PICU admission could not be calculated in the model.
Comparison of RSV patients with and without nirsevimab administration in the first 48h from admission (univariate analysis).
| Variablea | Nirsevimab in first 48h(n=18) | No nirsevimab in first 48h(n=63) | OR (95% CI) |
|---|---|---|---|
| Male | 8/18 (44.4) | 43/63 (68.3) | 2.6 (0.92–7.83) |
| Age (months) | 2.38 (1.32–3.51) | 2.67 (1.43–4.27) | p=0.27 |
| Length of stay (days) | 6 (4–9) | 5 (3–7) | p=0.051 |
| Oxygen support | 17/18 (94.4) | 63/63 (100) | 0.14 (0.09–2.51) |
| Nasal cannula | 12 (70.6) | 31 (49.2) | 1.10 (0.31–3.94) |
| High-flow therapy | 5 (29.4) | 18 (28.6) | 2.4 (0.78–2.9) |
| CPAP and BiPAP | – | 14 (22.2) | 0.731 (0.633–0.843) |
| Apnea episodes | 2/10 (20) | 2/19 (10.5) | 2.1 (0.25–17.9) |
| Enteral nutritionb | 4/18 (22.2) | 29/58 (50.0) | 0.286 (0.084–0.972) |
| PICU admission | 0/18 (0) | 20/63 (31.7) | 0.705 (0.599–0.829) |
OR: odds ratio, CI: confidence interval. Significant results are in bold.
As far as we are concerned, this is the first study that describes the clinical effect of early nirsevimab administration during hospitalization in non-immunized infants with acute RSV bronchiolitis. Administration within the first two days of admission seems to be associated with a significant reduction in the need for nasogastric feeding. Despite the confidence interval is wide, we consider that this work shows a trend that should be corroborated in a larger, well-designed study, possibly in a clinical trial. Moreover, although none of these infants required positive-pressure oxygen therapy or PICU admission, the small sample size probably prevents finding significant results in the regression model for these variables. Nirsevimab administration was carried out to ensure passive immunization against RSV, but a possible effect as treatment has been observed. In spite of the fact that we did not observe a difference in the length of stay, the reduction in severity is an important goal. Generally, the management of acute bronchiolitis includes positive-pressure oxygen therapy when respiratory distress progresses [10] or with the presence of apnea or hypercapnia [11]. Nasogastric feeding is required for patients who cannot tolerate oral feeding [10,11]. PICU admission is indicated when respiratory failure, apnea with desaturation or severe impairment of general conditions occur [11]. In our study, both reference hospitals had similar PICU admission criteria, with no significant differences in the management of patients between them.
Nirsevimab is a monoclonal antibody that binds the F1 and F2 subunits of the RSV fusion (F) protein, locking it in a prefusion conformation to prevent viral entry into the host cell [12]. It has been approved by the EMA and recommended by several infectious disease societies to immunize neonates and infants [4,13] against RSV. There are two types of RSV, A and B, and no differences have been described in the effectiveness of nirsevimab between both subgroups [7]. Compared to palivizumab, the first monoclonal antibody approved for preventing bronchiolitis, they differ in the target protein, due to palivizumab binds to the fusion F protein site II epitope to stop viral replication [9,14]. Palivizumab has been evaluated as a treatment option for infants admitted with RSV acute bronchiolitis but did not demonstrate effectiveness [9].
The winter season of October 2023–March 2024 was the first in Spain with general administration of nirsevimab to infants under 6 months and children under 2 years with risk factors [4]. In our study, a significant decrease in hospitalization (64.5%) and RSV positivity (71.2%) was observed compared with the previous season (2022–2023). Ernst et al. described a similar reduction in hospitalization in Luxembourg (69% in infants under 6 months old [15]).
Although our study could not determine the immunization rate, other Spanish authors reported coverage rates of 87–92% [6–8,17]. The low administration rate in our study (42.6%) could suggest a bias because we were analyzing RSV-positive bronchiolitis, and it is expected to have more cases without nirsevimab in that group. Regarding the effectiveness of nirsevimab, authors describe 82–88.7% effectiveness against RSV-lower respiratory tract infections (LRTI) admissions [6–8] and 90% against RSV-associated hospitalization [5]. Coma et al. described an effectiveness of 87.6% and 90.1% in hospital and PICU admissions from October 2023 to January 2024 in Catalonia [18]. At the end of the campaign in Madrid, effectiveness was 87.6% in preventing hospital admission and 90.7% in avoiding the need for intensive care unit [16]. These results are also consistent with efficacy results of hospitalization for RSV-associated LRTI from the MELODY [11] and HARMONIE [19] clinical trials. We detected 21 RSV bronchiolitis cases despite immunization. The MELODY trial detected a relatively lower efficacy in infants 3 months old or younger at the moment of immunization [11].
Our study has several limitations. First of all, this is a retrospective observational study without the controlled design of a clinical trial with a post hoc analysis, so results must be taken with caution because we might have overestimated the effect of nirsevimab. Secondly, the different timing of nirsevimab administration depending on the hospital of admission could be another source of bias, which reinforces the need for proper studies. In third place, we did not establish the days of symptoms before hospitalization, an important factor that could impact the clinical course of bronchiolitis and the potential utility of nirsevimab in non-immunized infants with RSV LRTI.
In conclusion, despite our results seem promising because it highlights a possible therapeutic effect of nirsevimab, controlled clinical trials with a large sample size are needed to establish the real utility of early administration during an RSV infection as a treatment.
CRediT authorship contribution statementConceptualization, A.M.E, J.A.S.S, P.F.P and C.C; methodology, A.M.E, J.A.S.S and C.C; validation, A.M.E, R.J.G, F.B.A and C.C; formal analysis, J.A.S.S and C.C; investigation and data curation, E.S.C, P.F.P, B.S.R.L.T, M.T.P and S.A; writing – original draft preparation, A.M.E and J.A.S.S; writing – review and editing, A.M.E, J.A.S.S and C.C; visualization, A.M.E, R.J.G, F.B.A, M.L.G.G and C.C; supervision, A.M.E, J.A.S.S, R.J.G, M.L.G.G and C.C. All authors have read and agreed to the published version of the manuscript.
Declaration of generative AI and AI-assisted technologies in the writing processDuring the preparation of this work, the authors used ChatGPT 3.8 in order to improve readability and language. After using this tool, the authors reviewed and edited the content as needed and took full responsibility for the content of the publication.
FundingThis research received no funding.
Conflict of interestThe authors have no conflicts of interest to disclose.
Authors would like to acknowledge Dr Elisabeth Castaño-Guerra and Dr Alberto García-Salido for their help with statistical analysis.