Inhaled corticosteroids (ICS) are one of the most frequently prescribed treatments in the management of respiratory inflammatory diseases. Indeed, ICS are extensively used in both asthma1 and COPD (particularly those manifesting peripheral eosinophilia),2,3 and both of these conditions are highly prevalent in the general population.4,5 In the vast array of commercially available ICS, there is a high degree of variability among the various formulations, including different chemical and molecular compositions, inhalation devices, formulations of the compound (dry-powder, solution, metered-doses), particle sizes, and being either in isolation or combined with other pharmacological agents such as short-acting or long-acting bronchodilators.6
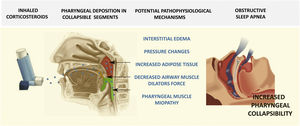
It has been estimated that around a billion people suffer from obstructive sleep apnea (OSA) globally.7 In this context, complex associations have been identified between OSA and other respiratory conditions that are usually treated with ICS. Such is particularly the case on the relationships between asthma and OSA. On the one hand, asthma and OSA are both extremely prevalent diseases and consequently their co-occurrence would be expected in a certain proportion of individuals.1,6,8 On the other hand, several studies have shown that the presence of OSA can lead to aggravation of asthma, possibly via the presence of rhinitis, obesity, or gastroesophageal reflux disease (GERD) among many other possible factors.9 A recent meta-analysis indicated that the prevalence of OSA in asthmatic patients revolves around 50% and that the odds of having OSA in an asthmatic individual are 2.64-fold higher than in non-asthmatic patients.10 Of note, similar findings have been consistently reported in asthmatic children, whereby the prevalence of OSA in persistent moderate asthmatics exceeds 60%, clearly a much higher prevalence than that recorded among non-asthmatic children.11,12 As a result, some authors postulate that the presence of underlying asthma could facilitate the emergence of OSA or enhance the severity of OSA.13 The current evidence to support such possibilities is not as robust in adults even though it has been extensively documented in pediatric patients.14,15 In adults, after adjusting for important confounders, it was reported that the presence of excessive daytime sleepiness (EDS) or habitual snoring among asthmatic patients was more likely.16 One of the potential mechanisms advanced as an explanation for these associations is the chronic administration of ICS in asthma.17–20 To this effect, studies both in animal models21 and in humans have reported that chronic treatment with ICS will induce a spectrum of alterations within the upper airway function that promotes its collapsibility. For example, ICS may lead to local interstitial edema of the upper airway walls, may promote changes in the critical collapsible pressures of the airway, enhance the deposition of adipose tissue within the pharyngeal muscles, reduce the force generated by the airway dilators, primarily the genioglossus, or induce a corticosteroid myopathy within the pharyngeal musculature (Fig. 1). All these consequences of chronic ICS therapy are a priori unrelated to the severity of the underlying asthma, but are more likely to become manifest with increasing ICS dosages. Thus, it is not inconceivable to advance the supposition of a biologically plausible role played by ICS (particularly high dose ICS) in promoting an overall myopathic and more collapsible pharyngeal airway segment that becomes manifest as OSA in the more susceptible patient.20 This situation would be remarkably similar to the frequently observed dysphonic effects of ICS as a consequence of the myopathy effect on the vocal cord muscles.22
Notwithstanding, although the clinical studies conducted to date point to similar directions than those raised above, they are hampered by substantial methodological limitations that preclude more definitive conclusions. For example, a retrospective population-based study from Taiwan that included nearly 40,000 individuals with and without asthma, the adjusted hazard ratio (aHR) for the presence of OSA was 1.33 (95%CI: 1.01–1.76) among those asthmatic patients treated with ICS versus those not receiving ICS.23 Similarly, Teodorescu and colleagues evaluated 244 patients with asthma (37% with habitual snoring, 40% at high-risk of OSA). In this cohort, the independent contributors to the presence of snoring included GERD, and use of ICS (OR, 2.66; 95%CI: 1.05–6.72). Similarly, high-risk of OSA was associated with asthma severity, GERD and ICS use (OR, 4.05; 95%CI: 1.56–10.53). Furthermore, dose-dependent relationships emerged between ICS dosage and both snoring and high-risk of OSA (p:0.004 and p:0.0006, respectively16).
Thus, if long-term ICS treatment induces upper airway collapsibility by one or more of the aforementioned mechanisms, this signifies that there is a substantial deposition of ICS in the upper airway walls that is capable of generating these biological and functional effects. As a corollary, any interventions aimed at reducing the deposition of ICS during routine chronic treatment of asthma should reduce the risk of upper airway dysfunction and of OSA in asthmatic patients. We are unaware of any extant studies that have assessed how improved inhalatory maneuvers or techniques that minimize ICS deposits in the upper airway wall result in lessened risk of OSA. Similarly, there are no comparative studies as to whether use of pro-ICS compounds, such as ciclesonide,24 that are activated only upon delivery to the lung and are not active in the upper airway mucosa affect the upper airway. Despite the lack of such studies, it is now well established that ICS preparations that are delivered as ultrafine particles lead to marked reductions in the deposition of the corticosteroid in the upper airway while also being accompanied by reduced side-effects.25 In this context, Henao et al. performed the only available study that tackles these issues. This was a retrospective study that included 29,816 patients with asthma. These investigators found that ICS users were more likely to suffer from OSA compared to those patients not receiving ICS (aOR, 1.11; 95%CI: 0.78–1.58). However, among those patients receiving ICS in formulations consisting of ultrafine particles, such increased risk was not present. The obvious limitations of this study, namely being retrospective and relying on correlational databases, do not allow for more definitive conclusions.17 Yet, these findings are provocative enough to justify more methodically adept studies in light of the importance and disease burden of both asthma and OSA and their potential interactions as far as multisystem morbidities. Thus, clinical trials aimed at uncovering the potential contribution of long-term ICS treatment to the risk of OSA and its magnitude are needed. If such increased risk is indeed confirmed and corroborates the biological plausibility advanced above, it will be then critical to establish whether ultrafine ICS particle formulations of pro-ICS preparations eliminate this risk, with the attendant implications for clinical guidelines related to asthma management in OSA patients. Independently, we can already advocate for improved asthmatic patient education aimed to optimize the use of inhalers such as to reduce the proportion of ICS being deposited in the upper airway. Similarly, it seems prudent to advocate and perform sleep studies among asthmatic patients who chronically receive high doses of ICS or those who manifest localized side-effects such as snoring or voice alterations to evaluate for the presence of OSA.
Conflict of interestsNone of the authors declares any conflict of interest.