Diffuse panbronchiolitis (DPB) is a bronchiolar inflammatory process that is chronic, progressive, and seen almost exclusively in Asian patients. It usually occurs between the second and fifth decades of life, and two-thirds of the patients have no history of smoking. Symptoms include exertional dyspnea, cough with expectoration that in many cases exceeds 50ml/day, sinus affectation, and dry rales. The typical radiological findings are bilateral diffuse micronodules, “tree-in-bud” pattern, and on occasion there may be bronchiectasis. The main histologic finding is the accumulation of foamy macrophages in the walls of respiratory bronchioles.1 The levels of cryoagglutinins, IgA, and RF are frequently altered and the lung function tests may show obstructive, restrictive, or mixed pattern.
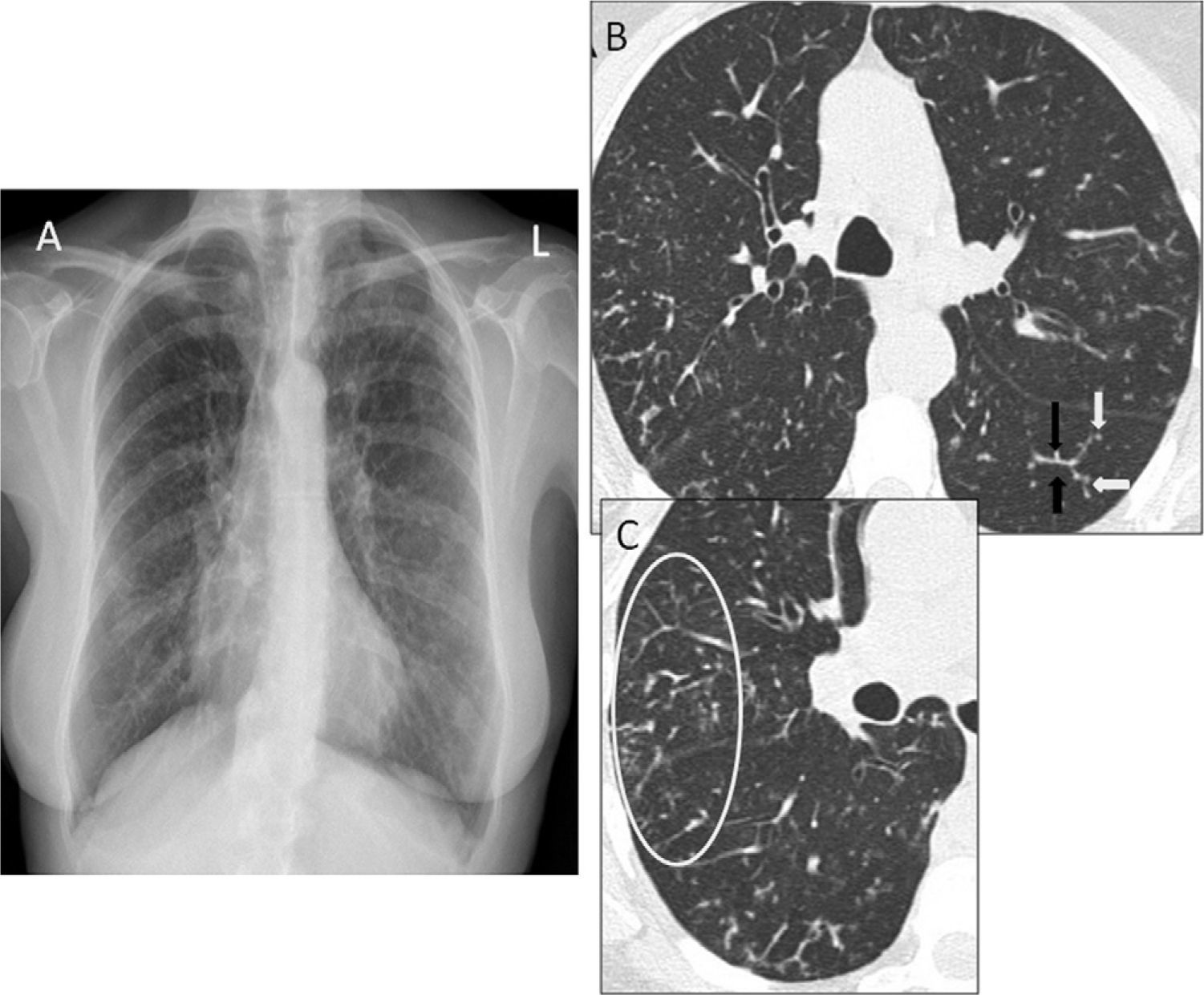
We present the case of a 56-year-old Caucasian woman who has never been a smoker and has no notable family, work, or pharmacological history. For the past 2 years, she has experienced daily persistent productive cough and dyspnea upon moderate exertion. Physical examination revealed bibasilar dry rales. The basic analytical data were normal, except for rheumatoid factor 53IU/ml (normal <20IU/ml) and immunoglobulin A 484mg/dl (normal 82–453mg/dl). Chest radiograph (Rx) showed small bilateral nodular opacities and thoracic high-resolution computed tomography (HRCT) demonstrated “tree-in-bud” pattern (Fig. 1). Spirometry revealed a mild restrictive pattern and the 6-min walk test showed a drop in arterial oxygen saturation from 98% to 90%. Echocardiogram was normal. Bronchioloalveolar lavage showed 87% neutrophils, CD4/CD8 ratio 1.16, and transbronchial biopsy showed no alterations. Given the lack of a definitive diagnosis, a surgical lung biopsy (SLB) was taken. The anatomopathologic study revealed chronic inflammation located mainly in the respiratory bronchioles, with lymphocytes, plasma cells, and foamy histiocytes; in the bronchiolar lumen, neutrophils were predominant. All these histologic data fit the diagnosis for DPB. Treatment was initiated with oral clarithromycin (250mg every 12h) and during the first 6 months of follow-up, the patient has presented good tolerance of the treatment prescribed, although the initial symptoms persist.
(A) Posteroanterior chest radiograph where diffuse bilateral micronodular interstitial pattern can be observed. (B) Thoracic HRCT image showing “tree-in-bud” pattern, consisting of branching structures (black arrows) and buds (white arrows). (C) A wider view with abundant peripheral buds (circle, C).
Even though the etiology and natural history of this disease are not precisely known, the genetic susceptibility has been associated with human leukocyte antigens (HLA)-B54 and -A11.2 In the medical literature reviewed, there is a small series of cases originating in Western countries of Europe, Australia, and America.3–5 The diagnostic clinical criteria established by the Committee on Diffuse Lung Diseases of the Japanese Ministry for Health and Well-Being are only applicable for Asian populations. In western countries, where this disease is very rare and the clinicians are not accustomed to treating it, SLB is usually necessary. The current treatment available includes macrolides (erythromycin and clarithromycin), which should be administered for at least 2 years.6
In short, the diagnosis of DPB is usually difficult in many patients due to the non-specific clinical and radiological characteristics, in addition to the fact that it is not suspected due to its infrequency in Western countries. Nevertheless, it should be kept in mind when evaluating patients without a history of smoking who present with exertional dyspnea, chronic productive cough, and tree-in-bud pattern on chest HRCT. We would like to end by highlighting the fact that, after an exhaustive review of the medical literature, this is the first reported case of DPB originating in Spain, and it is one of the very uncommon cases described in Europe.
Please cite this article: Urbano Aranda Y, et al. Panbronquiolitis difusa. Una rara enfermedad en países occidentales. Arch Bronconeumol. 2012; 48: 184-5.