The management of chronic obstructive pulmonary disease (CODP) is a challenge, particularly for patients with a low FEV1 where long-acting muscarinic antagonist (LAMA), or a combination of a long-acting β2 agonist (LABA) plus an inhaled corticosteroid (ICS) therapies are not able to reduce exacerbation rates. In recent years, triple therapy in one inhaler, consisting of a fixed-dose combination of an ICS, a LAMA, and a LABA, has been proposed for patients with COPD in whom earlier regimens have failed to reduce the rate of exacerbation. To date, six randomized clinical trials have been published with promising results showing altogether a reduction of exacerbation rates or a lower mortality when triple therapy was compared with other alternatives. Nevertheless, the methodology (i.e. inclusion criteria, follow-up), the treatments compared, the endpoints considered, and co-variates collected were different among them, making difficult to decide which triple therapy should fit better for which type of patient.1 We would like to raise some relevant points that a pneumologist should consider when analysing the real usefulness of such treatments for patients.
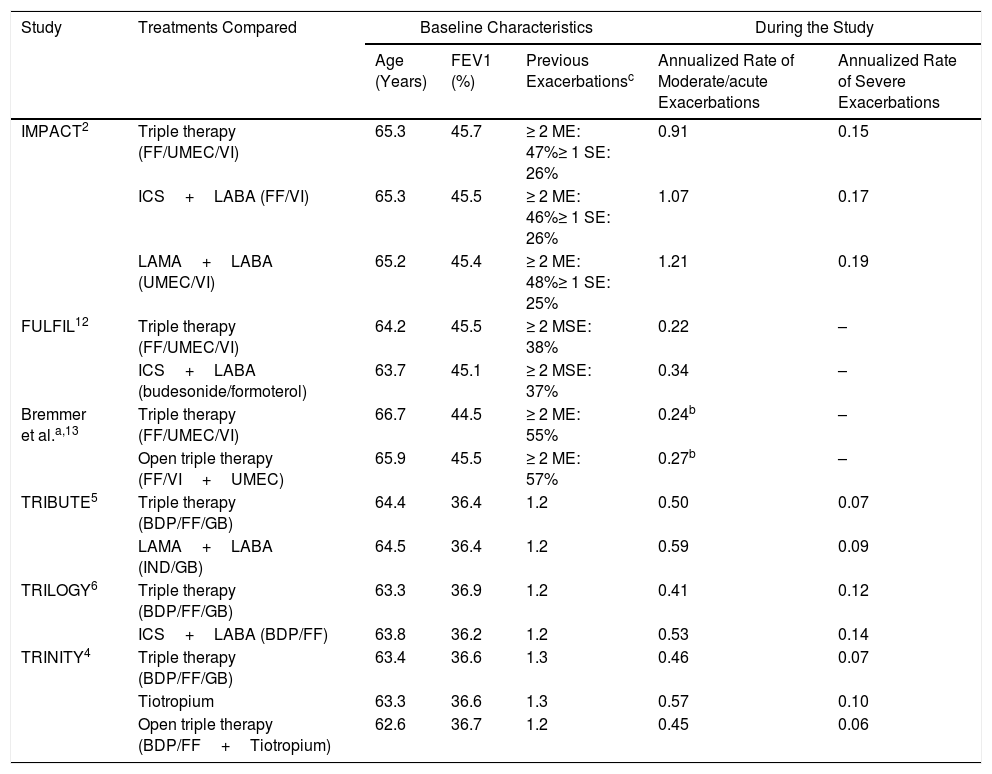
First, the number of annualized moderate/severe and severe exacerbation rates differed among the trials (Table 1). The IMPACT study2 reported the highest rate of moderate/severe exacerbations (2–4 times higher), irrespective of the treatment regimen. These differences have been attributed to an abrupt cessation of ICS in the IMPACT study.3 Nevertheless, a comparison of other characteristics of the six studies is somehow paradoxical. Although the COPD Assessment Test questionnaire score was very similar among all studies, the forced expiratory volume in 1s was considerably lower for the TRINITY,4 TRIBUTE,5 and TRILOGY6 studies. Therefore, the IMPACT trial reported an increased number of exacerbations, with greater preservation of lung function. In addition to the different inclusion criteria, it is also possible that moderate exacerbations were recorded in a different manner by the IMPACT study. This hypothesis might be supported by the fact that the differences in severe exacerbations (an objective criterion, because it entails hospital admission) were much lower than those observed for moderate exacerbations. Moreover, the TRIBUTE, TRINITY, and TRILOGY studies do not precisely define a moderate exacerbation.4–6
Comparison of Triple Therapy Studies Based on the Rate of Exacerbation and Treatment Received.
| Study | Treatments Compared | Baseline Characteristics | During the Study | |||
|---|---|---|---|---|---|---|
| Age (Years) | FEV1 (%) | Previous Exacerbationsc | Annualized Rate of Moderate/acute Exacerbations | Annualized Rate of Severe Exacerbations | ||
| IMPACT2 | Triple therapy (FF/UMEC/VI) | 65.3 | 45.7 | ≥ 2 ME: 47%≥ 1 SE: 26% | 0.91 | 0.15 |
| ICS+LABA (FF/VI) | 65.3 | 45.5 | ≥ 2 ME: 46%≥ 1 SE: 26% | 1.07 | 0.17 | |
| LAMA+LABA (UMEC/VI) | 65.2 | 45.4 | ≥ 2 ME: 48%≥ 1 SE: 25% | 1.21 | 0.19 | |
| FULFIL12 | Triple therapy (FF/UMEC/VI) | 64.2 | 45.5 | ≥ 2 MSE: 38% | 0.22 | – |
| ICS+LABA (budesonide/formoterol) | 63.7 | 45.1 | ≥ 2 MSE: 37% | 0.34 | – | |
| Bremmer et al.a,13 | Triple therapy (FF/UMEC/VI) | 66.7 | 44.5 | ≥ 2 ME: 55% | 0.24b | – |
| Open triple therapy (FF/VI+UMEC) | 65.9 | 45.5 | ≥ 2 ME: 57% | 0.27b | – | |
| TRIBUTE5 | Triple therapy (BDP/FF/GB) | 64.4 | 36.4 | 1.2 | 0.50 | 0.07 |
| LAMA+LABA (IND/GB) | 64.5 | 36.4 | 1.2 | 0.59 | 0.09 | |
| TRILOGY6 | Triple therapy (BDP/FF/GB) | 63.3 | 36.9 | 1.2 | 0.41 | 0.12 |
| ICS+LABA (BDP/FF) | 63.8 | 36.2 | 1.2 | 0.53 | 0.14 | |
| TRINITY4 | Triple therapy (BDP/FF/GB) | 63.4 | 36.6 | 1.3 | 0.46 | 0.07 |
| Tiotropium | 63.3 | 36.6 | 1.3 | 0.57 | 0.10 | |
| Open triple therapy (BDP/FF+Tiotropium) | 62.6 | 36.7 | 1.2 | 0.45 | 0.06 | |
ICS, inhaled corticosteroids; LAMA, long-acting muscarinic antagonist; LABA, long-acting β2 agonist; FF, Fluticasone furoate; UMEC, Umeclidinium; VI, vilanterol; BDP, Beclomethasone Dipropionate; FF, Formoterol Furoate; GB, Glycopyrronium Bromide; IND, Indacaterol; NR, not reported; ME, moderate exacerbations; SE, severe exacerbations.
Second, only the IMPACT study measured mortality; and a post hoc analysis of the TRIBUTE, TRINITY, and TRILOGY studies showed their mortality rate.7 One-year mortality ranged from 1.9% (fixed triple therapy arm in the TRINITY study)4 to 2.9% (LAMA+LABA arm in IMPACT) for all studies.2 These one-year mortalities are significantly low for COPD patients of such characteristics, when compared with other evidence. The UK-COPD audit reported 3.8% in-hospital mortality.8 The European COPD Audit reported a 90-day mortality of 10.8%, following hospital admittance,9 and that reported by the Spanish COPD Audit was 6.9%.10 Therefore, one-year mortality rates for patients with severe COPD enrolled in triple therapy studies should be higher. A younger inclusion age may partly account for these differences, since patients enrolled in triple therapy studies were aged between 63 and 65 years, and the average age of the audit studies was between 71 and 75 years. However, these age differences might not fully explain the low mortality observed in these clinical trials.
The third aspect is related to follow-up and the number of participants at risk for exacerbation. The data from triple therapy studies depict that approximately 30%–40% of patients attending follow-up appointments are “at risk”, i.e. being followed in the cohort, by the end of follow-up (52 weeks). This loss of patients cannot be explained by the lost-to-follow-up proportion. We understand that a patient suffering from moderate or severe exacerbation is “at risk” of suffering from another episode after resolution of the first. The low mortality rate observed and that 85% of all participants reached the end of the study, as reported, could be potentially explained by the fact that only the first exacerbation was considered and that the real annual exacerbation rate was not calculated. If this is true, key information regarding the mechanism of action of triple therapy for COPD might be lost.
We would like to stress the need of further studies with triple therapy, which should consider the following inclusion and exclusion criteria: all patients should have a CAT>10 and>10 pack-years; patients should have had a COPD hospital admission in the last 3–6 months (severe exacerbation), to improve comparability and avoid differences on what is understood by moderate exacerbation (where subjective assessment may be present); patients should represent average age of patients with those COPD characteristics (i.e. 70 years old or at least some stratification by age should be present to have representative age groups of young, average, and old COPD patients); men and women should be equally represented – in the UK more women than men are admitted due to COPD; previous triple therapy treatment should be excluded, because randomization will mean that some patients already with triple therapy would receive an a priori worse treatment (double therapy); the study should be triple blind, with a masked statistician. Regarding the follow-up, at least two years would be necessary to show long-term effects of triple therapy (mainly for the ICS treatment), or at least analysis of severe exacerbations and mortality rate should be presented at two-years.11 The analysis of these two endpoints is quite easy if electronic clinical records are used.
To conclude, although triple therapy may be adequate for the treatment of patients with COPD in whom other treatment regimens have failed to improve the clinical presentation, there are some relevant questions awaiting answers, which prevent the generalization of these results to average COPD populations. New studies, with longer follow-up, homogenous inclusion and exclusion criteria, particularly regarding previous treatments, and with a better representativeness of a standard COPD patient with a worse lung function are still needed.
Conflicts of interestDr. Ruano-Ravina has nothing to disclose. Dr. Lopez-Campos reports personal fees and non-financial support from AstraZeneca, grants, personal fees and non-financial support from Boehringer Ingelheim, grants, personal fees and non-financial support from Chiesi, personal fees and non-financial support from CSL Behring, grants, personal fees and non-financial support from Esteve, personal fees and non-financial support from Ferrer, grants, personal fees and non-financial support from GebroPharma, grants, personal fees and non-financial support from GlaxoSmithKline, grants, personal fees and non-financial support from Grifols, grants, personal fees and non-financial support from Menarini, grants, personal fees and non-financial support from Novartis, grants, personal fees and non-financial support from Rovi, grants, personal fees and non-financial support from Teva, outside the submitted work. Dr. Fernández-Villar reports personal grants fees and non-financial support from AstraZeneca, grants, personal fees and non-financial support from GlaxoSmithKline, grants, personal fees and nonfinancial support from Boehringer Ingelheim, grants, personal fees and non-financial support from Roche, personal fees and non-financial support from Chiesi, personal fees and non-financial support from Esteve, personal fees and non-financial support from Novartis, grants, personal fees and non-financial support from Rovi, personal fees and non-financial support from GebroPharma, personal fees and non-financial support from Bial and grants from Menarini, outside the submitted work.