Nontuberculous mycobacterial pulmonary disease (NTM-PD) is considered an emerging disease with increasing incidence. The importance of NTM infections lies in the fact that they require long treatments, with at least three drugs, and with poorer outcomes than tuberculosis, implying both high costs and a significant impact on patient quality of life.1–4
Environmental conditions as well as individual susceptibility play an important role in the development of NTM-PD, with differences in disease prevalence and great variability in the causative species by geographical area. There are also important clinical differences between the NTM species in their aggressiveness and response to treatment.
Well-known studies compare the clinical relevance of the isolation of different NTM species in the northern Europe and the USA, but to our knowledge, no similar studies have been conducted in southern Europe.5,6 Taking into account the marked geographic differences in the distribution of NTM, we undertook a detailed study of all the NTM isolates in respiratory samples in our area. The main aim was to compare the pathogenicity of the NTM isolated (percentage of first isolates from respiratory samples associated with true lung disease according to the 2020 ATS criteria). As secondary objectives, we sought to investigate associations of risk factors, and clinico-radiological characteristics with specific NTM species and to examine the clinical course with and without treatment.
This was a multicentre observational study, based on retrospective data from 2012 to 2017 and prospective data collected between 2018 and 2020, in four tertiary hospitals in the Basque Country in Spain. We included all patients aged≥18 years with NTM isolation from respiratory samples. Data on NTM isolates were supplied monthly by the microbiology services within the tuberculosis control program. The study was approved by the local ethics committees and conducted in accordance with the Declaration of Helsinki for research in humans.
We recorded data on patient demographic characteristics, medication and baseline comorbidities. We also collected data on symptoms, microbiological data, and radiological findings. If the patients met the criteria for NTM pulmonary disease (ATS 2020), we studied the treatment prescribed (initiation or not, its duration) and outcomes. Patients were followed up for at least 2 years and we analyzed mortality and relapse.
Final outcomes were classified according to the Nontuberculous Mycobacteria Network European Trials Group treatment outcome definitions consensus: (1) cure: three consecutive negative cultures with clinico-radiological improvement; (2) clinical cure: sustained improvement in symptoms, without evidence of three negative cultures; (3) treatment failure: persistence of positive cultures with the causative species after ≥12 months of antimycobacterial treatment.7
Chi-square or Fisher's exact tests were used for comparing categorical data. Differences were considered statistically significant when p<0.05. When clustering was in more than two groups, to compare two by two, thresholds for significance were adjusted for multiple comparisons using Tukey's method or Benjamini–Hochberg's method.
NTM were isolated from 433 patients, of whom 134 met the criteria for NTM pulmonary disease. Of these, we analyzed the patients with the most common nontuberculous mycobacteria species in depth.
Overall, the median age was 65 years, although patients with Mycobacterium abscessus were significantly younger. 47% of the patients were male with no differences by species. The main risk factors and radiological findings are summarized in Table 1. Clinical symptoms were very non-specific with cough and expectoration in >90% of cases and are not listed in the table. Only 14 patients (10%) with NTM-PD did not have any known risk factors.
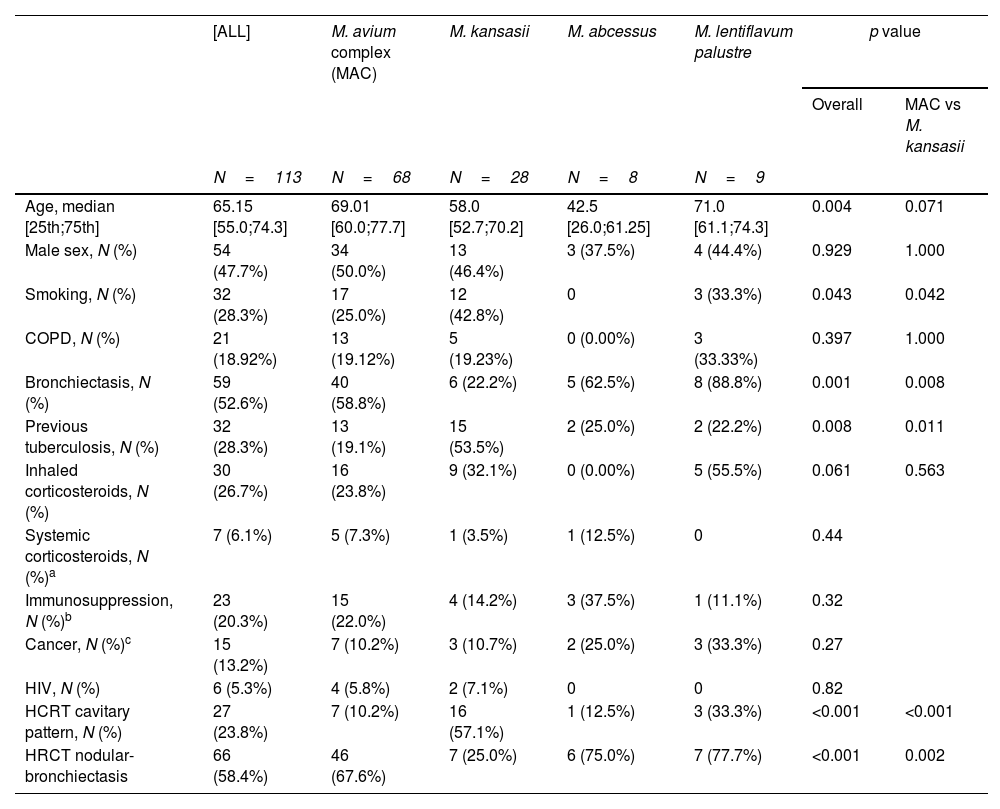
Risk factors and high-resolution computed tomography (HRCT) patterns by mycobacterial species.
| [ALL] | M. avium complex (MAC) | M. kansasii | M. abcessus | M. lentiflavum palustre | p value | ||
|---|---|---|---|---|---|---|---|
| Overall | MAC vs M. kansasii | ||||||
| N=113 | N=68 | N=28 | N=8 | N=9 | |||
| Age, median [25th;75th] | 65.15 [55.0;74.3] | 69.01 [60.0;77.7] | 58.0 [52.7;70.2] | 42.5 [26.0;61.25] | 71.0 [61.1;74.3] | 0.004 | 0.071 |
| Male sex, N (%) | 54 (47.7%) | 34 (50.0%) | 13 (46.4%) | 3 (37.5%) | 4 (44.4%) | 0.929 | 1.000 |
| Smoking, N (%) | 32 (28.3%) | 17 (25.0%) | 12 (42.8%) | 0 | 3 (33.3%) | 0.043 | 0.042 |
| COPD, N (%) | 21 (18.92%) | 13 (19.12%) | 5 (19.23%) | 0 (0.00%) | 3 (33.33%) | 0.397 | 1.000 |
| Bronchiectasis, N (%) | 59 (52.6%) | 40 (58.8%) | 6 (22.2%) | 5 (62.5%) | 8 (88.8%) | 0.001 | 0.008 |
| Previous tuberculosis, N (%) | 32 (28.3%) | 13 (19.1%) | 15 (53.5%) | 2 (25.0%) | 2 (22.2%) | 0.008 | 0.011 |
| Inhaled corticosteroids, N (%) | 30 (26.7%) | 16 (23.8%) | 9 (32.1%) | 0 (0.00%) | 5 (55.5%) | 0.061 | 0.563 |
| Systemic corticosteroids, N (%)a | 7 (6.1%) | 5 (7.3%) | 1 (3.5%) | 1 (12.5%) | 0 | 0.44 | |
| Immunosuppression, N (%)b | 23 (20.3%) | 15 (22.0%) | 4 (14.2%) | 3 (37.5%) | 1 (11.1%) | 0.32 | |
| Cancer, N (%)c | 15 (13.2%) | 7 (10.2%) | 3 (10.7%) | 2 (25.0%) | 3 (33.3%) | 0.27 | |
| HIV, N (%) | 6 (5.3%) | 4 (5.8%) | 2 (7.1%) | 0 | 0 | 0.82 | |
| HCRT cavitary pattern, N (%) | 27 (23.8%) | 7 (10.2%) | 16 (57.1%) | 1 (12.5%) | 3 (33.3%) | <0.001 | <0.001 |
| HRCT nodular-bronchiectasis | 66 (58.4%) | 46 (67.6%) | 7 (25.0%) | 6 (75.0%) | 7 (77.7%) | <0.001 | 0.002 |
Of the 134 patients who met disease criteria, only 98 received treatment (73%), with significant differences between the different NTMs as shown in Table 2. 88% of the treatments were in accordance with current guidelines. The most common reasons for not prescribing treatment were clinical stability (n=22), older age (n=5), patient refusal (n=5), or the existence of another health problem with a poor prognosis (n=9).
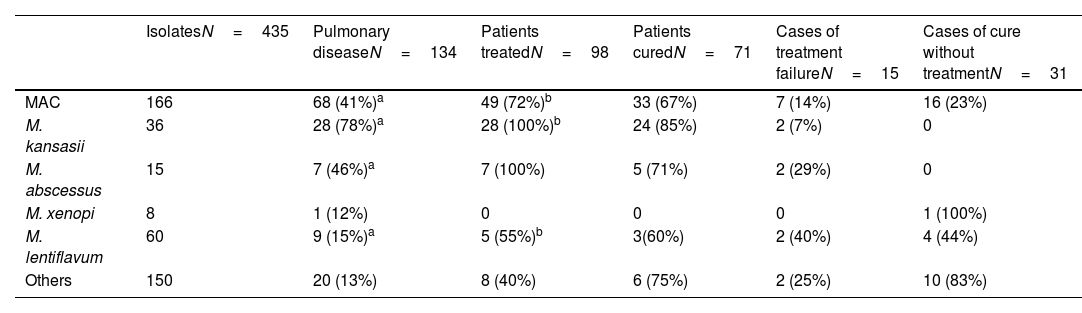
Pathogenicity, treatment, and outcomes.
| IsolatesN=435 | Pulmonary diseaseN=134 | Patients treatedN=98 | Patients curedN=71 | Cases of treatment failureN=15 | Cases of cure without treatmentN=31 | |
|---|---|---|---|---|---|---|
| MAC | 166 | 68 (41%)a | 49 (72%)b | 33 (67%) | 7 (14%) | 16 (23%) |
| M. kansasii | 36 | 28 (78%)a | 28 (100%)b | 24 (85%) | 2 (7%) | 0 |
| M. abscessus | 15 | 7 (46%)a | 7 (100%) | 5 (71%) | 2 (29%) | 0 |
| M. xenopi | 8 | 1 (12%) | 0 | 0 | 0 | 1 (100%) |
| M. lentiflavum | 60 | 9 (15%)a | 5 (55%)b | 3(60%) | 2 (40%) | 4 (44%) |
| Others | 150 | 20 (13%) | 8 (40%) | 6 (75%) | 2 (25%) | 10 (83%) |
Between-species differences in the rate of disease (percentage of isolates considered clinically relevant) that remained significant after correction for multiple comparisons were:
- MAC compared to M. kansasii and M. lentiflavum.
- M. kansasii with MAC, M. abcessus and M. lentiflavum.
- M. abcessus with M. kansasii and M. lentiflavum.
The response rate differed between the species (from 82% to 60%), with a treatment failure rate of 15%.
The novelty of this study is that it offers a global vision of the pathogenicity and treatment response of the most common NTM in our area and underlines the differences between species.
MAC is the species found most frequently, as is the case in most of the world8–10 and the percentage of isolates considered clinically relevant in our setting is also similar to that described by other authors (41% vs rates varying from 27% to 56% in northern Europe and USA5,6,11,12). In our study, 72% of the MAC patients who met the disease criteria were treated, a slightly higher rate than in other studies.13,14
Moreover, a considerable percentage of these untreated patients (23%), despite meeting disease criteria, met clinical criteria for cure. Unfortunately, the subsample is too small for statistical analysis, but relatively high rates of recovery with no treatment have also been reported previously, reaching as high as 40–50% in the aforementioned studies.13,14
Mycobacterium lentiflavum, a lesser-known species, was the second most common mycobacterium isolated but showed very low pathogenicity, as described by other authors.15 On the other hand, its response to treatment was poor.
In our setting, Mycobacterium kansasii is by far the most pathogenic species but has an excellent response to treatment as described elsewhere.16 The second most pathogenic species in our series is M. abscessus, with a treatment response rate of 72%, notably higher than in other studies, probably due to a significant proportion of cases corresponding to M. abscessus massiliensis which responds better to treatment.17
Underlying lung diseases are a risk factor for the development of pulmonary disease, and we found differences between mycobacterial species, with bronchiectasis being an important risk factor in MAC and M. abscessus (especially in patients with cystic fibrosis in the latter), while in M. kansasii, having previously had tuberculosis is the main risk factor. COPD appears as a risk factor in less than 20% of patients with no differences between the species. Inhaled corticosteroids, a risk factor often considered avoidable in COPD patients, was present in just 26% of our patients, a much lower rate than reported in the literature,18 and there were no significant differences between species.
Finally, regarding radiological characteristics, our data are consistent with previous studies. Specifically, there are significant differences between the different mycobacteria, with a nodular-bronchiectatic pattern being the most common in all species except M. kansasii, with which over 57% of cases present a cavitary pattern.
Some limitations of the present study should be addressed. First, its retrospective part implies some potential weaknesses, but we did not detect differences between retrospective and prospective data. Additionally, patients were followed up for only 2 years, which may be insufficient due to the slow progressive nature of the disease.
To conclude, MAC was disease causing in less than half of the isolates, with a spontaneous cure rate of 20% and a treatment failure rate of 14%, while M. kansasii was pathogenic in nearly 80% of isolates with an excellent response to treatment. Our results show that the clinical relevance and treatment outcome differ markedly between mycobacterial species, with a broad spectrum of disease with different expectations regarding response to treatment and clinical course, and hence, different criteria and treatment durations may be needed.
Authors’ contributionsETH and LAU take the responsibility of the manuscript as a whole. ETH, LAU, JAGF, and MVLA conceived and designed the study. ETH, LAU, JAGF, MVLA, BOUA and NOL enrolled patients and collected and compiled data. BSZ performed the statistical analysis. ETH and PAM analyzed and interpreted the data. ETH and LAU wrote the manuscript. JAGF, MVLA, BOUA commented and revised the report. All authors read and approved the final manuscript.
Availability of data and materialThe data that support the findings of this study are available on request from the corresponding author.
Ethics approval and consent to participateThe Biomedical Research Ethics Committee of Euskadi (PI2019-001) approved this study.
Consent for publicationAll authors have accepted the publication of the manuscript.
Funding sourcesGrant from the Department of Health of the Basque Government2018111098.
Conflict of interestsThe authors declare they have no conflict of interest.