The goals of COPD treatment are to improve symptoms and decrease future risks such as exacerbations, lung function decline and mortality. Despite receiving maximal inhaled treatment, many COPD patients continue to exacerbate. This highlights the need for novel therapeutic approaches. There is now positive clinical trial data supporting the use of monoclonal antibody treatments in COPD. There remain challenges to define responder populations for each monoclonal antibody class on the basis of clinical characteristics and biomarkers. There are considerable future opportunities, including the targeting of disease activity with monoclonal antibodies at earlier stages in the natural history of COPD in order to slow the rate of disease progression. Using monoclonal antibodies during an exacerbation as a targeted treatment also hold potential. Monoclonal antibody treatments have the potential to change the landscape of COPD management.
Persistent small airway inflammation is a hallmark feature of chronic obstructive pulmonary disease (COPD), resulting in airway remodelling and destruction [1]. Airway inflammation in COPD is complex, involving a network of different cell types and mediators, and heterogeneous as the components involved vary from one individual to another [2]. An example of this heterogeneity is the variable presence of protease mediated alveolar destruction (emphysema) [3]. Additionally, it is now recognised that a subgroup of COPD patients have type-2 (T2) inflammation [4].
Underlying inflammatory processes in COPD give rise to physiological abnormalities, including airflow obstruction and gas trapping, and clinical characteristics such as dyspnoea, cough and sputum production [5]. Many patients also suffer with exacerbations, which are rapid symptom worsenings that are often caused by respiratory tract infections [5]. As inflammation is heterogeneous in COPD, then so too is the physiological and clinical presentation.
The goals of COPD treatment are to alleviate symptoms and decrease the future risk of exacerbations, lung function decline and mortality [5]. Inhaled corticosteroids (ICS) are the most widely used anti-inflammatory drug in COPD, reducing exacerbation rates and improving quality of life in selected patients identified using clinical characteristics (a history of exacerbations) coupled with evidence of T2 inflammation based on blood eosinophil counts (BECs) [6,7]. Despite optimisation of inhaled treatment regimes, many COPD patients continue to exacerbate, highlighting the need for novel therapeutic approaches [8].
Monoclonal antibody (Mab) treatments have been used successfully in many chronic inflammatory diseases [9,10]. Mabs are highly targeted treatments requiring a precision medicine approach to identify patients who are most likely to gain benefit [11,12]. We provide a narrative review of the evidence for the efficacy of Mabs in COPD with the challenge of identifying responder subgroups based on clinical and/or biomarker characteristics. We also consider evolving concepts and opportunities for the use of Mabs in COPD, including the targeting of disease activity and the use of Mabs during exacerbations.
Inflammation in COPDCigarette smoke activates pattern recognition receptors on epithelial cells resulting in the secretion of inflammatory mediators [13] and release of damage associated molecular patterns (DAMPS) including epithelial alarmins [14]. Inflammatory mediators and DAMPs upregulate the recruitment and activation of neutrophils and macrophages [14] enabling the clearance of particulate matter from the airways (phagocytosis) [15]. However, persistent smoking causes inflammation caused by innate immune dysregulation [16] which can cause pathological changes such as goblet cell hyperplasia, reduced cilia function, increased airway thickening (due to the deposition of extracellular matrix proteins) and alveolar attachment destruction [17–20]. Progression of these pathological changes causes poorly reversible airflow obstruction, the cardinal physiological feature of COPD, along with emphysema [21]. Epithelial barrier dysfunction and reduced immune cell mediated anti-microbial defence increase susceptibility to infections, further propagating inflammatory cascades [22,23].
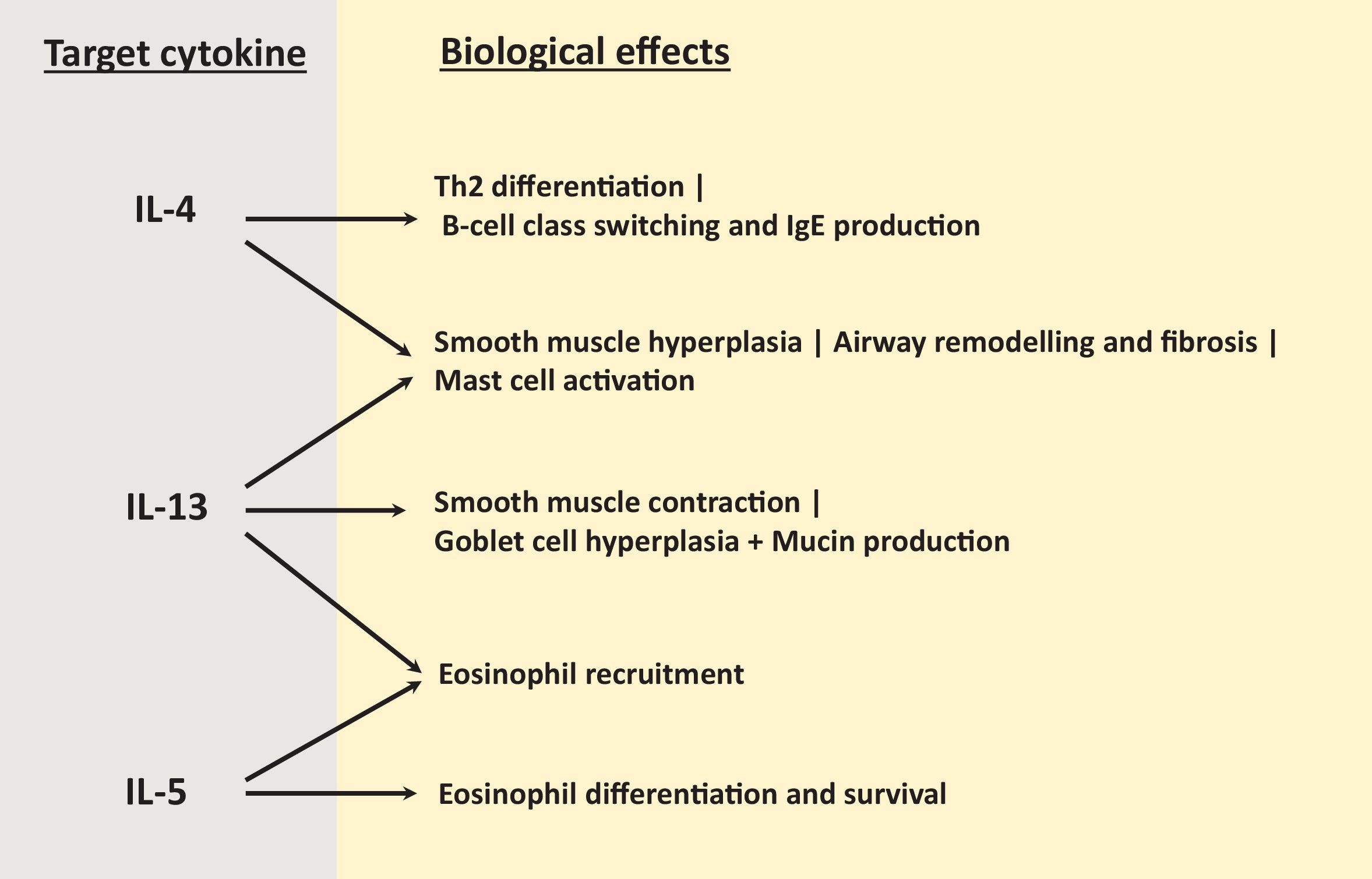
T2 inflammation is characterised by increased numbers of eosinophils, T-helper 2 (Th2) cells, type 2 innate lymphoid (ILC2) cells and the cytokines IL-4, IL-5, and IL-13 [12]. The major roles of these T2 cytokines relevant to COPD are explained in Fig. 1; IL-4 promotes Th2 differentiation [24], IL-5 controls eosinophil differentiation and survival [25] while IL-13 is involved in smooth muscle hyperplasia and contraction, mucus hypersecretion and airway remodelling [26]. There is now consistent evidence that a COPD sub-group have elevated pulmonary eosinophil counts with upregulation of T2 mediators including IL-13 and IL-13 inducible genes such as C-C motif chemokine ligand 26 [27–30]. Importantly, increased T2 inflammation in COPD patients is often present without any history of asthma or evidence of allergy [27,31], with the T2 gene expression profile in COPD differing from asthma [30]. These observations highlight that the presence of T2 inflammation in COPD patients should not be oversimplified as the presence of asthma [4].
Higher BEC are used as a biomarker to identify COPD patients with T2 inflammation [27,29–31]. Higher BEC are associated with faster lung function decline in COPD patients and the development of COPD in smokers [32,33], implicating T2 inflammation as a cause of COPD progression.
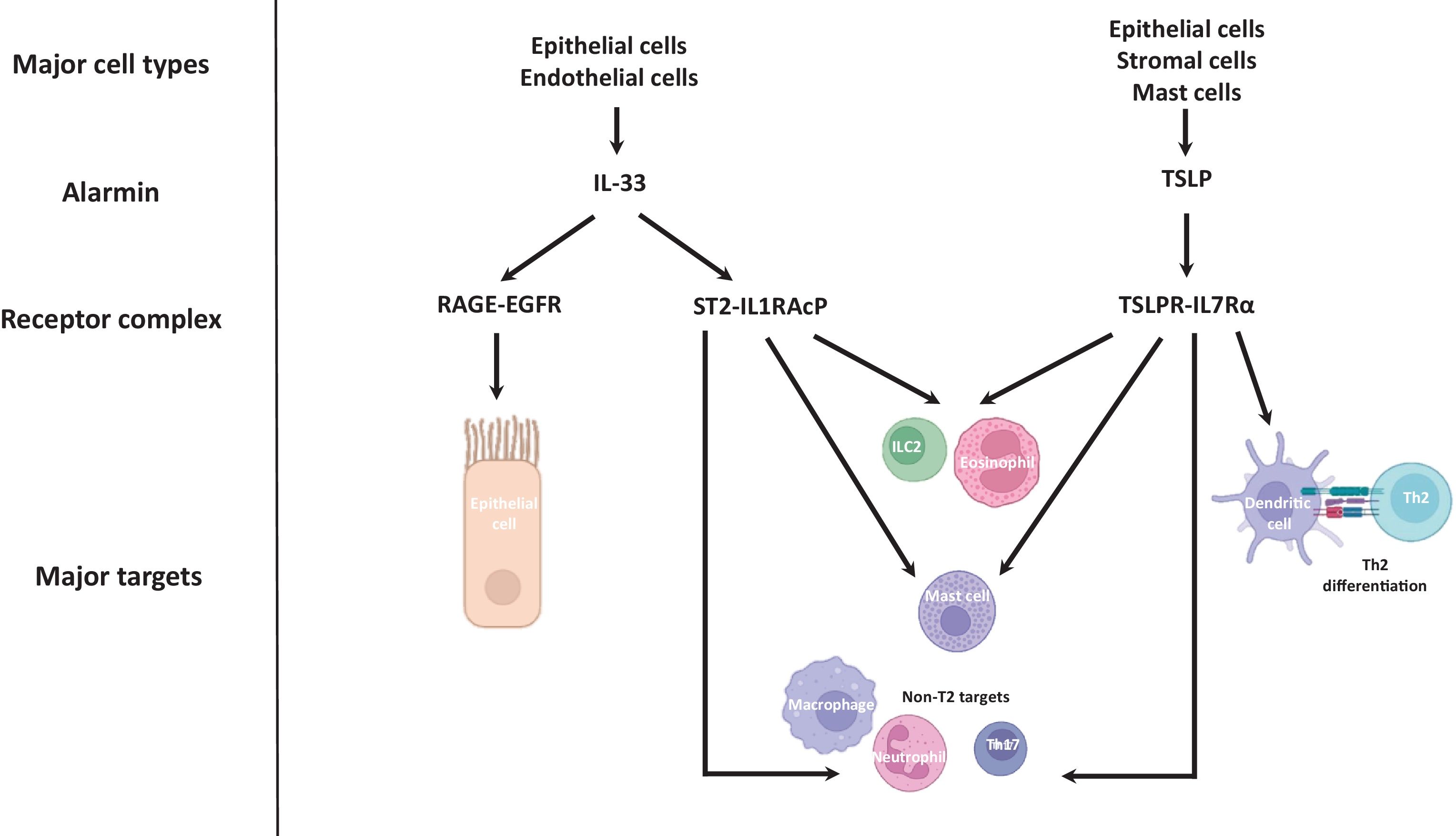
The alarmins, including thymic stromal lymphopoietin (TSLP) and IL-33, are rapidly released cytokines which upregulate T2 and non-T2 immune responses [34] (Fig. 2 shows cellular sources and major actions of IL-33 and TSLP). TSLP is mainly released by epithelial cells and stromal cells, but also by mast cells, and binds to the TSLPR-IL-7Rα receptor complex to promote differentiation of naïve CD4+ T-cells into Th2 cells through regulation of dendritic cell expression of OX40 ligand [34,35]. TSLP also stimulates T2 cytokine production from ILC2 cells and mast cells [36,37], including IL-5 which leads to eosinophil recruitment [38]. TSLP can regulate neutrophil function, for example by induction of CXCL8 to facilitate chemotaxis [39]. In COPD patients, TSLP protein levels are increased in sputum supernatants and BAL fluid, and the number of cells expressing TSLP mRNA in the epithelial layer of bronchial biopsies are increased in COPD patients versus controls [40].
IL-33 and TSLP signalling relevant to COPD pathophysiology. The major cellular sources of IL-33 are epithelial cells and endothelial cells. IL-33 triggers goblet cell hyperplasia via the RAGE-EGFR receptor complex, and activates T2 and non-T2 immune responses via the ST2-IL1RAcP receptor complex. The major cellular sources of TSLP are epithelial cells, stromal cells and mast cells. TSLP activates T2 and non-T2 immune responses via the TSLPR-IL7Rα receptor complex, including dendritic cell mediated Th2 differentiation. Artwork produced with the use of BioRender.
Epithelial basal cells are major producers of IL-33. This alarmin acts through the suppressor of tumourigencity 2 (ST2)-IL1R accessory protein (IL1RAcP) receptor complex expressed on several cell types including mast cells, eosinophils, Th2 cells and ILC2 cells, facilitating the release of T2 mediators [34,41,42]. Sputum IL-33 protein levels are increased in COPD patients compared to controls and levels are correlated with blood and sputum eosinophil numbers [43,44]. IL-33 also invokes non-T2 responses such as neutrophil extracellular trap formation [45]. Oxidation of IL-33 results in ST2 independent induction of goblet cell hyperplasia and impairment of epithelial wound closure [46]; these effects are mediated through the receptor for advanced glycation end products (RAGE)/epidermal growth factor receptor (EGFR) on epithelial cells [46].
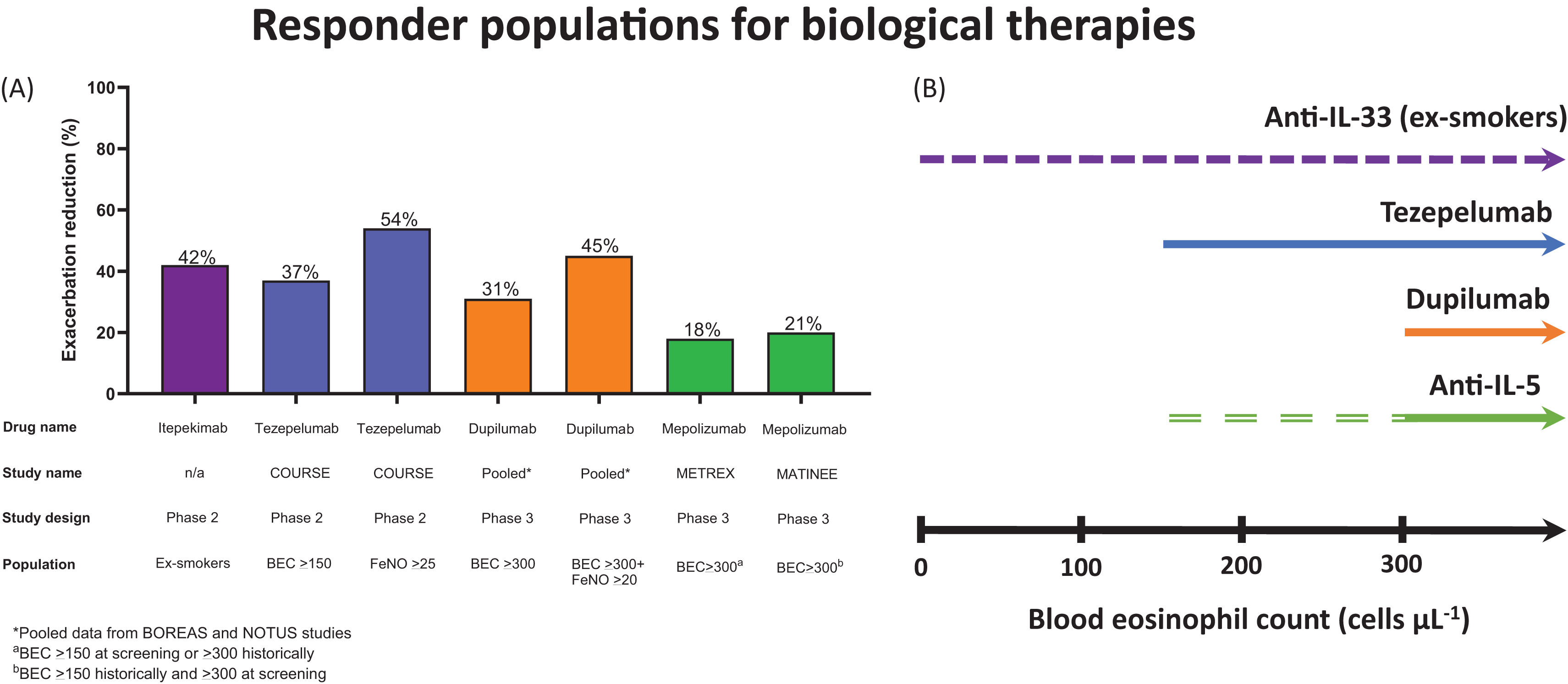
Clinical trials in COPD with MabsFig. 3a shows the characteristics of the responder populations in placebo controlled phase 2 and 3 COPD studies of Mabs that have reported positive data regarding exacerbation prevention. Dupilumab is a Mab that targets the IL-4Rα, thereby blocking the actions of both IL-4 and IL-13. Dupilumab has shown clinical benefits in diseases characterised by T2 inflammation, notably atopic dermatitis and severe asthma. Two recent phase 3 studies (BOREAS and NOTUS; n=939 and 935, respectively) with similar designs demonstrated the efficacy of dupilumab in COPD patients with T2 inflammation defined as BECs≥300cells/μL at screening [47,48], a history of chronic bronchitis and 2 moderate or 1 severe exacerbation in the previous year while using inhaled triple therapy. A pooled analysis of these studies reported that dupilumab (compared to placebo) reduced the rate of moderate or severe exacerbations by 31.3% (incidence rate ratio 0.687; p<0.0001), caused a greater improvement in pre-bronchodilator FEV1 at week 12 and week 52 (mean differences 0.083L and 0.073L, respectively; both p<0.0001) and improved quality of life at week 4 to week 52 (SGRQ score mean differences −2.16 and −3.4, respectively; both p<0.001) [49]. There are multiple potential mechanisms by which dupilumab can improve lung function, including the attenuation of IL-13 induced smooth muscle constriction and hyperplasia and blockade of IL-13 induced mucus production [26,50]. The effect of dupilumab on exacerbations was greater (45% reduction) in patients with higher FeNO levels (>20ppb), suggesting that FeNO could be an alternative biomarker (to BEC) to predict treatment response [49].
(A) An overview of the effect of monoclonal antibodies on the rate of moderate or severe exacerbation reduction (%) in specific COPD populations from randomised controlled trials. (B) Responder populations according to blood eosinophil counts (BEC) in COPD patients. Dupilumab has shown efficacy in individuals with eosinophils>300cells/μL at screening, but a greater response in individuals who also have FeNO>20ppb. The purple dashed line indicates the efficacy of anti-IL-33 biologicals may be similar across a range of BEC. The green dashed line indicates anti-IL5 biologicals have some efficacy in COPD patients with BEC>150cells/μL at screening with increasing efficacy with BEC>300cells/μL (solid green line).
Mabs that target IL-5 signalling to reduce pulmonary eosinophilic inflammation have been investigated in clinical trials in COPD patients with a history of exacerbations. The initial phase 3 studies with mepolizumab focused on a primary analysis population with BECs≥150cells/μL at screening or ≥300cells/μL in the previous year [51] while the relevant criterion for benralizumab was BEC≥220cells/μL at screening [52]. There was inconsistent evidence for exacerbation prevention across these studies, but greater effects were observed at higher BEC (i.e. ≥300cells/μL) [51,52]. The METREX phase 3 study defined eosinophilic patients as BEC≥150cells/μL at screening or BECs≥300cells/μL within the previous year, with the primary endpoint analysis showing a reduction in moderate to severe exacerbations in this population after treatment with mepolizumab (18% reduction, p=0.036). The METREO study, in a similar population, was negative for the same outcome. A subsequent study with mepolizumab included COPD patients with BEC≥300cells/μL at screening plus BECs≥150cells/μL within the previous year in order to enrich the population towards more consistent evidence of eosinophilic inflammation [53]. Mepolizumab reduced the rate of moderate and severe exacerbations (rate ratio, 0.79; p=0.01), although there were no improvements in other clinical outcomes.
The effects of Mabs that target alarmins have been investigated in COPD patients. A phase 2 study investigated tezepelumab which binds to TSLP; the enrolled patients had ≥2 exacerbations while using triple therapy (n=333) [54]. The primary analysis showed that tezepelumab reduced exacerbations by 17% which did not reach significance (p=0.10), but pre-defined subgroup analysis demonstrated increased effects on exacerbations, lung function and SGRQ in patients with BEC≥150cells/μL, with the greatest benefits at ≥300cells/μL [54]. The pattern of increasing clinical effects at higher BEC with tezepelumab has been observed in severe asthma [55]. While TSLP can potentially influence T2 and non-T2 pathways [56], these results in asthma and COPD indicate a greater effect on T2 inflammation.
The results of phase 2 trials of Mabs that target IL-33 signalling have shown early evidence of efficacy in specific subgroups. Itepekimab and tozorakimab target IL-33 [57,58] while astegolimab targets ST2 [59]. Itepekimab (n=343) reduced exacerbation rates in ex-smokers (42% reduction; p=0.006) with no effect in current smokers [57]. This treatment difference may relate to previous reports of reduced IL-33 levels in current smokers, possibly explained by current smoking increasing basal cell differentiation [41]; basal cells are a major source of IL-33 secretion in the lungs and IL-33 expression reduces upon differentiation [42].
Tozorakimab treatment over 6 months (n=135) showed greater effects on FEV1 and COPDcompEX, which is a composite measure of symptom worsenings plus exacerbations and peak expiratory flow measurements, in the subgroup with ≥2 exacerbations in the previous year [58]. Itepekimab and tozorakimab both reduced BEC and treatment effects on lung function were greater at higher baseline BEC [57,58]. Taken together, these observations demonstrate inhibition of type 2 inflammation with associated clinical benefit. However, higher BEC were not associated with an increased treatment effect on exacerbation prevention. This may suggest that IL-33 blockade prevents exacerbations related to both T2 and non-T2 mechanisms, while lung function benefits are more related to inhibition of T2 mechanisms. Additionally, an exploratory sub-study provided preliminary evidence that tozorakimab reduced mucus plugging [58]. As mucus plugging is associated with worse clinical outcomes including exacerbations and mortality [60], this could be an important finding related to inhibition of IL-33 signalling through the RAGE/EGFR pathway [46].
While astegolimab had no effect on exacerbations in a small study (n=81), there was an improvement in quality of life and some evidence of increased efficacy in individuals with lower BEC [59]. The ongoing phase 3 studies with Mabs that target the IL-33 pathway are expected to provide more clarity on differential effects according to clinical characteristics, such as current smoking status, and the degree of T2 inflammation assessed using BEC.
Disease activityAcross different diseases, chronic inflammation can cause end organ tissue damage resulting in permanent disability. Low disease activity (LDA) is a treatment target in many chronic inflammatory diseases; this describes the situation where treatment has suppressed inflammation thereby reducing the risk of disease progression or acute events such as flares of auto-immune diseases [61]. A closely related concept is remission where LDA has been achieved and the burden of symptoms is low or absent [62]. Remission is more difficult to achieve when there is significant end organ damage resulting in chronic symptoms but more feasible if intensive treatment is started earlier in the natural history of the disease [63].
Applying the concept of LDA as a treatment target in COPD has challenges. While C-reactive protein is an easily measurable disease activity biomarker in rheumatoid arthritis, the equivalent does not exist for COPD. Consequently, exacerbations are used as a measure of disease activity, but these events are actually a consequence (or clinical outcome) of active inflammation. Furthermore, remission is an unrealistic goal in COPD because late diagnosis remains common, as permanent end organ damage causes chronic symptoms.
The clinical trials with dupilumab enrolled COPD patients with a history of multiple exacerbations; over half of the patients who received dupilumab did not experience an exacerbation over the 1-year treatment period [47,48]. Using exacerbations as a marker of disease activity categorises these patients as achieving LDA. In support, there were improvements in lung function and quality of life in many individuals compatible with a lack of disease progression in a LDA state [47,48].
The concept of LDA as a treatment target has improved clinical outcomes in other diseases, reducing flares and disease progression [61,62]. The future use of Mabs in COPD may evolve to a similar concept. Future work is needed to develop a definition of LDA in COPD. The development of biomarkers to monitor disease activity would help in this regard, alongside better measures of disease progression beyond FEV1 such as computerised tomography imaging. It has been proposed that the term “stable” can be used when LDA is achieved in clinical practice; this can be a short- or long-term goal for COPD treatment [64].
Early COPD treatmentEmerging paradigms from rheumatology underscore the value of initiating targeted biologic therapy early to modify disease trajectory and prevent irreversible tissue damage. In rheumatoid arthritis and ankylosing spondylitis [11], early blockade of key cytokines halts radiographic progression and preserves function—even in minimally symptomatic patients—thereby establishing a precedent for similar strategies in COPD. Large cohort studies have shown that the presence of higher BEC in younger smokers or COPD patients is associated with more rapid lung function decline [33,65]. This implicates T2 inflammation in the progression of COPD and offers an opportunity to intervene with disease modifying treatments in biomarker defined subgroups at an early stage of disease. Targeting IL-13 has potential in this context, as this cytokine is involved in airway remodelling [26]. Furthermore, eosinophil-derived IL-13 could play a role as a mediator of alveolar destruction [66]. Doyle et al. used murine models to show that IL-13 secreted by recruited eosinophils drives emphysematous septal loss, while genetic or antibody-mediated IL-13 blockade attenuates airspace enlargement and preserves alveolar architecture [67]. These findings support the hypothesis that early neutralisation of IL-13 using Mabs such as dupilumab could arrest disease progression.
Anti-alarmin Mabs may also be useful as early interventions. In a murine model of chronic airway injury, IL-33 overexpression led to eosinophil recruitment, mucus hypersecretion, and alveolar wall destruction reminiscent of early emphysema, whereas IL-33-deficient mice were protected from these features [68], suggesting that IL-33 blockade may be able to forestall small-airway remodelling and parenchymal destruction. Effective deployment of early biologics in COPD pivots on precise endotype identification. While BECs remains a convenient surrogate to identify individuals with T2 inflammation, it does not directly quantify IL-13 or IL-33 activity. Surrogate markers such as serum periostin and fractional exhaled nitric oxide (FeNO) may enrich for IL-13–driven phenotypes [8]. Moreover, sputum IL-33 levels [69], once standardised, could stratify patients for IL-33–targeted therapy. Integrating multi-omic profiling—encompassing transcriptomic signatures of airway cells and proteomic analysis of bronchoalveolar lavage fluid—will further refine selection criteria and identify those most likely to benefit from early biologic intervention.
Biologics for acute treatment in COPDCOPD exacerbations are marked by sudden worsening of respiratory symptoms and systemic inflammation, frequently triggered by infections or environmental exposures [70]. Standard management is still associated with significant relapses and mortality [71], highlighting the urgent need for new therapies to improve patient outcomes. In this regard, Mabs have been studied during exacerbations.
The ABRA phase 2 study enrolled 158 adults with acute asthma or COPD exacerbations and BECs≥300cells/μL at the time of exacerbation [72]. Participants received either (1) prednisolone 30mg once daily for five days (PRED group), (2) a single 100mg subcutaneous injection of benralizumab (BENRA group), or (3) benralizumab plus prednisolone (BENRA+PRED group). Co-primary endpoints were proportion of treatment failures over 90 days and total visual analogue scale (VAS) symptoms at day 28. At 90 days, treatment failure occurred in 74% of the PRED group versus 45% in the pooled-BENRA groups (odds ratio 0.26; p=0.0005). By day 28, the benralizumab arms achieved a VAS reduction.
A single-centre, phase 2 study evaluated mepolizumab 100mg initiated at discharge in COPD patients hospitalised with an exacerbation and a BEC≥300cells/μL at any time in the preceding 12 months [73]. Patients receiving a single mepolizumab dose did not experience a significant reduction in 90-day rehospitalization or death, although a trend to fewer moderate and severe exacerbations was noted.
These findings could establish proof-of-concept for targeting of eosinophilic inflammation during COPD exacerbations. Rapid phenotypic stratification remains a barrier; timely eosinophil quantification and drug delivery in emergency settings are prerequisites for Mab administration. Development of point-of-care eosinophil assays and streamlined pathways for on-site subcutaneous injection will be critical. Moreover, evaluation of cost-effectiveness, optimal dosing regimens, and integration with bronchodilator and antibiotic protocols is needed.
The pharmacological evolution of Mabs in COPDThe positive efficacy data in phase 2 and 3 Mab studies paves the way for the development of new Mabs within the same classes but designed to either enhance potency or duration of action [74]. Whether increases in potency can also increase clinical efficacy remains to be seen, as the Mabs currently being studied may already be at the top of the dose response curve for clinical outcomes. However, longer duration of action may have practical benefits to patients and healthcare providers by reducing the dosing frequency.
Fig. 3 shows the precision medicine evidence for responder subgroups with different Mabs. Bispecific Mabs that combine two of these targets have been developed [75]; these may increase efficacy within a defined group (e.g. individuals with T2 inflammation) and/or broaden the target population.
ConclusionsThe positive clinical trial evidence for Mab treatment in COPD heralds the start of an optimistic era for COPD management. There remain challenges to define the responder populations for each Mab, with biomarker development still needed for this purpose. As Mabs target disease activity in COPD, there is the potential to treat at an early stage to modify the natural history; this is a priority area for evidence generation with the potential to change the landscape of COPD management.
Artificial intelligence involvementNo artificial intelligence software or tool was used to produce this article.
Funding of the researchDave Singh is supported by the National Institute for Health Research (NIHR) Manchester Bio-medical Research Centre (BRC). The authors would like to acknowledge the North West Lung Centre Charity for supporting this project. This report is independent research and the views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Conflicts of interestDS has received consultancy fees from Adovate, Aerogen, Almirall, Apogee, Arrowhead, AstraZeneca, Bial, Boehringer Ingelheim, Chiesi, Cipla, CONNECT Biopharm, Covis, CSL Behring, DevPro Biopharma LCC, Elpen, Empirico, EpiEndo, Genentech, Generate Biomedicines, GlaxoSmithKline, Glenmark, Kamada, Kinaset Therapeutics, Kymera, Menarini, MicroA, OM Pharma, Orion, Pieris Pharmaceuticals, Pulmatrix, Revolo, Roivant Sciences, Sanofi, Synairgen, Tetherex, Teva, Theravance Biopharma, Upstream and Verona Pharma.
AMB has nothing to disclose.
AH has nothing to disclose.
BAN reports grants and personal fees from GSK, grants, personal fees and non-financial support from AstraZeneca, personal fees and non-financial support from Boehringer Ingelheim, personal fees and non-financial support from Chiesi, grants, personal fees and non-financial support from Laboratorios Menarini, personal fees from Bial, personal fees from Zambon, personal fees from MSD, personal fees from PulmonX corporation and personal fees from Sanofi, outside the submitted work.