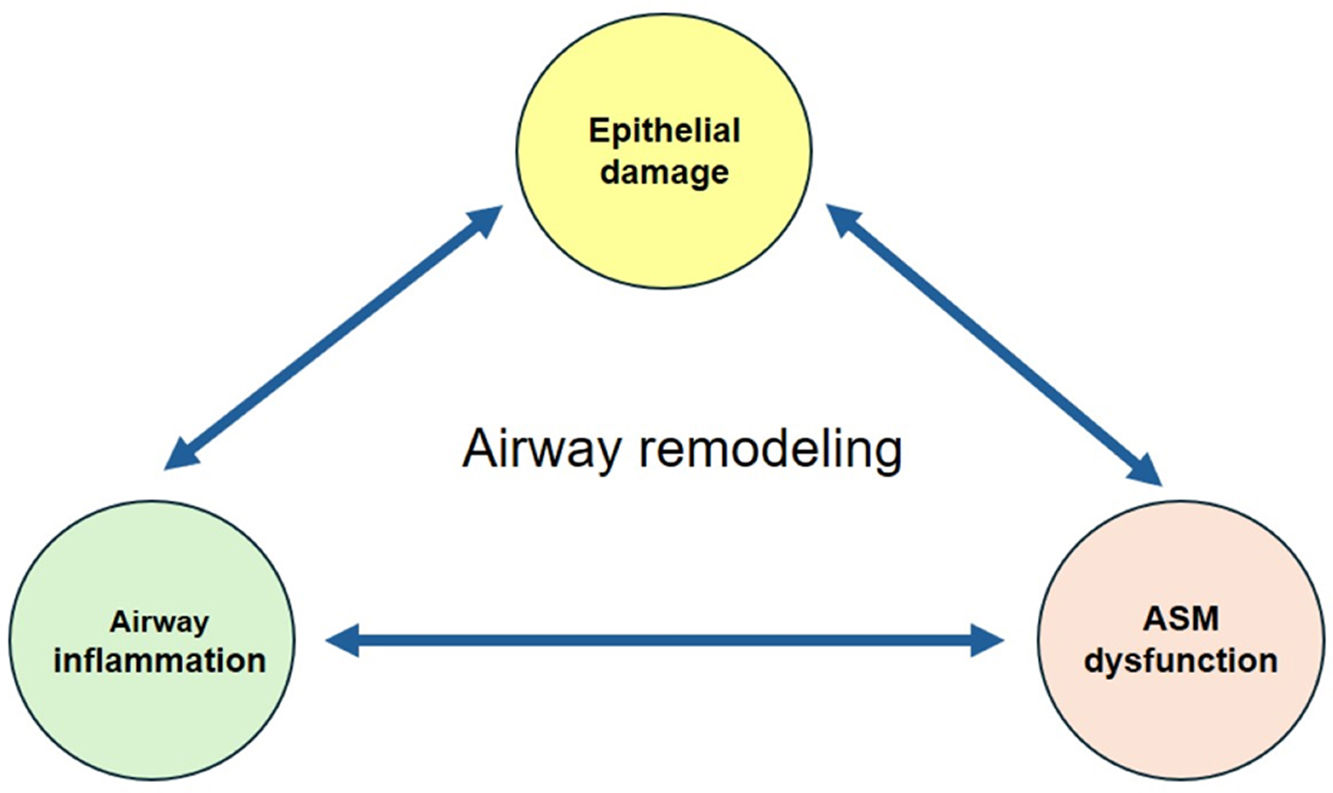
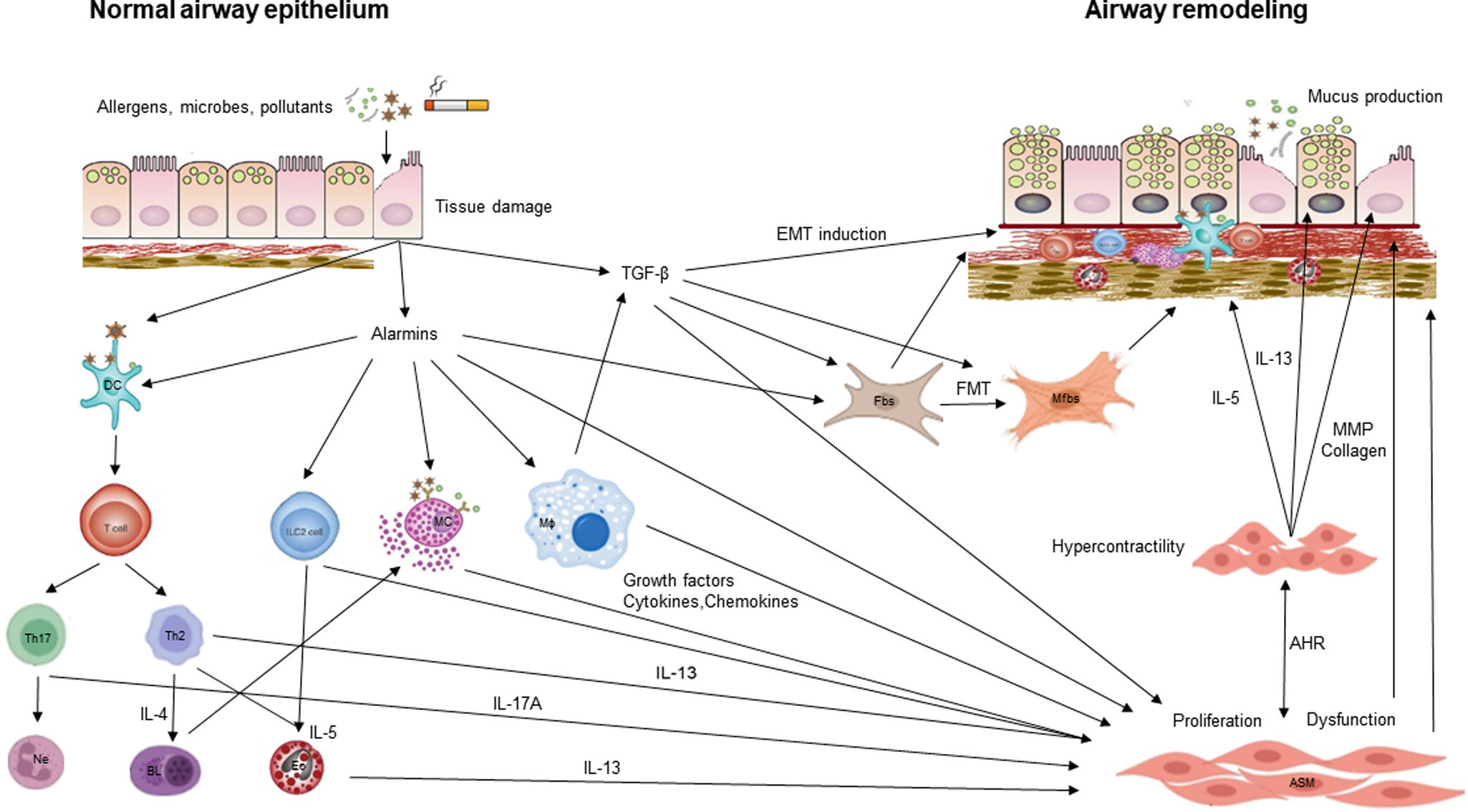
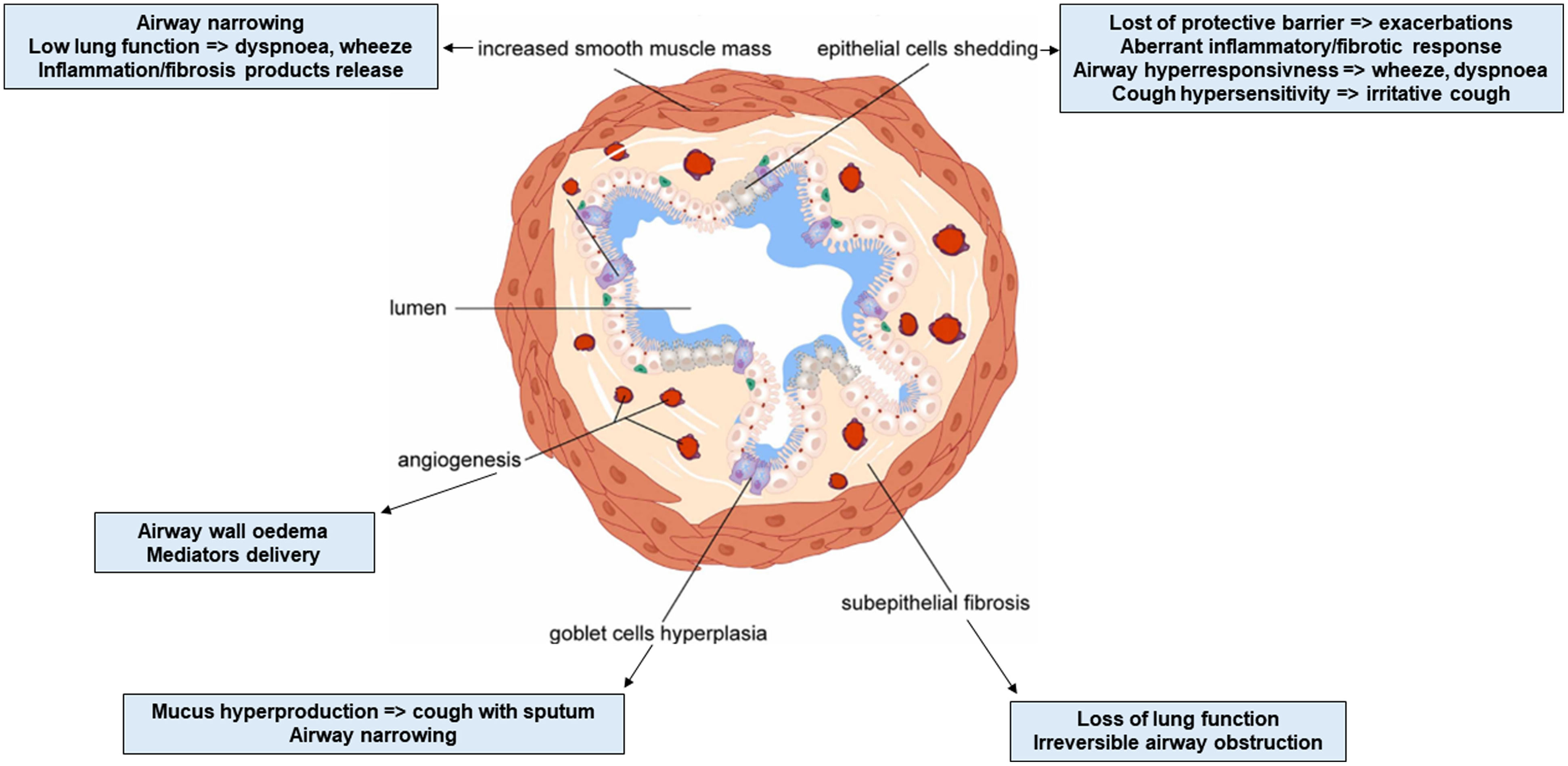
Airway remodeling (AR) with chronic inflammation, are key features in asthma pathogenesis. AR characterized by structural changes in the bronchial wall is associated with a specific asthma phenotype with poor clinical outcomes, impaired lung function and reduced treatment response. Most studies focus on the role of inflammation, while understanding the mechanisms driving AR is crucial for developing disease-modifying therapeutic strategies.
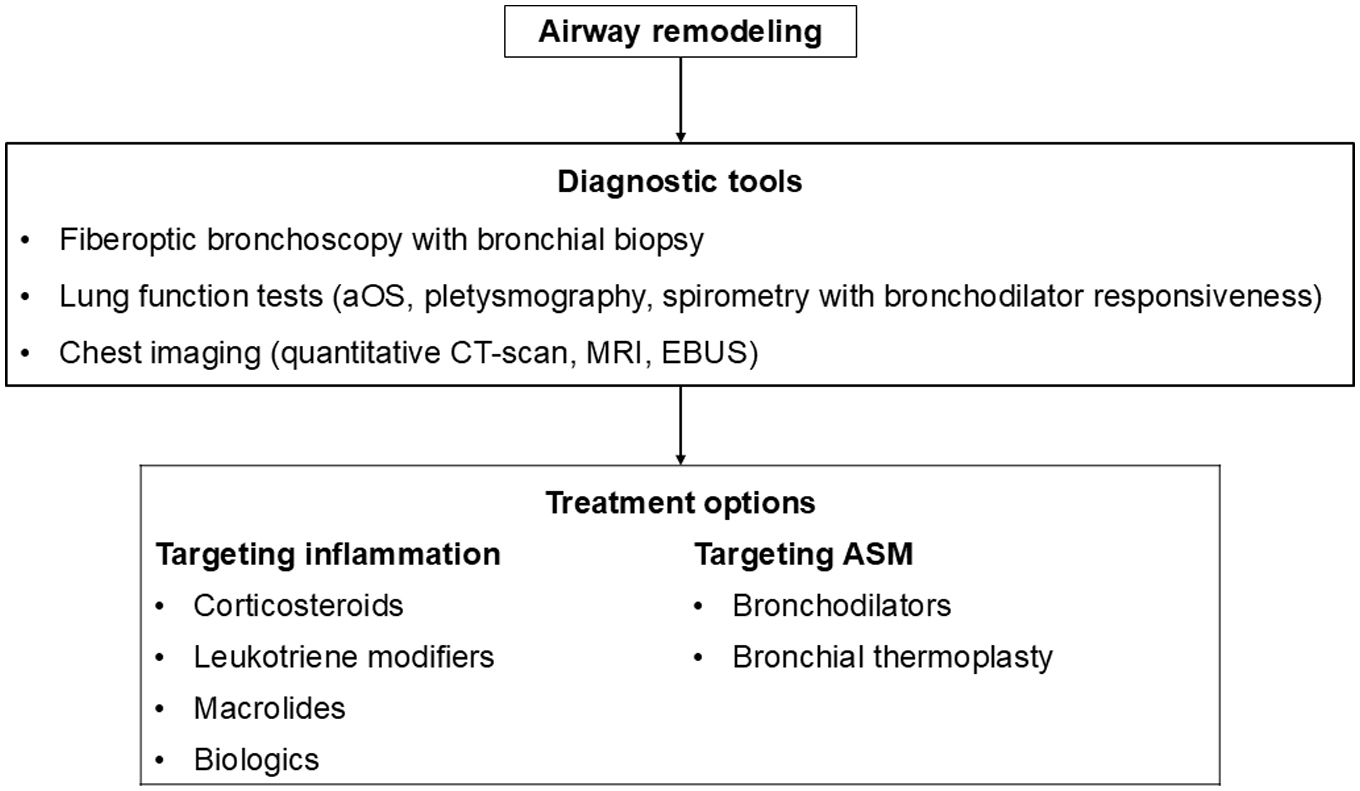
This review paper summarizes current knowledge on the mechanisms underlying AR, diagnostic tools, and therapeutic approaches. Mechanisms explored include the role of the resident cells and the inflammatory cascade in AR. Diagnostic methods such as bronchial biopsy, lung function testing, imaging, and possible biomarkers are described. The effectiveness on AR of different treatments of asthma including corticosteroids, leukotriene modifiers, bronchodilators, macrolides, biologics, and bronchial thermoplasty is discussed, as well as other possible therapeutic options.
AR poses a significant challenge in asthma management, contributing to disease severity and treatment resistance. Current therapeutic approaches target mostly airway inflammation rather than smooth muscle cell dysfunction and showed limited benefits on AR. Future research should focus more on investigating the mechanisms involved in AR to identify novel therapeutic targets and to develop new effective treatments able to prevent irreversible structural changes and improve long-term asthma outcomes.