In neuromuscular diseases (NMD), respiratory failure due to progressive diaphragmatic weakness is a leading cause of morbidity and mortality [1]. Nocturnal hypoventilation often precedes daytime respiratory failure, making early detection crucial. Non-invasive ventilation (NIV) is the gold standard treatment, improving symptoms, quality of life, and survival [2]. Early initiation of NIV, as soon as nocturnal hypoventilation is identified, may prevent acute hypercapnic episodes [3].
Although partial arterial pressure of carbon dioxide (PaCO2) >45mmHg in arterial blood gases (ABG) is the current gold standard to define hypoventilation, ABG provide only a momentary snapshot and may not reflect nocturnal PaCO2 trends. Nocturnal pulse oximetry (NPO) is easy to use but may underestimate hypoventilation [4]. Transcutaneous CO2 monitoring (TcPCO2) is more accurate for detecting nocturnal hypercapnia [5] but remains underused due to cost, limited availability, technical failure, and interpretation variability.
Recent data suggest that a measured venous total CO2 concentration (MVtCO2) >27mmol/L could serve as a surrogate marker of nocturnal hypoventilation in NMD [6]. Since nocturnal hypercapnia may cause residual metabolic alkalosis during the day, elevated MVtCO2 may help detect early ventilatory failure in NMD.
This monocentric retrospective study aimed to evaluate the predictive value of MVtCO2 for identifying nocturnal or diurnal hypoventilation and determining the need for NIV in a cohort of NMD patients with suspected diaphragmatic weakness.
Adult NMD patients referred for elective transcutaneous oxicapnography between January 2016 and May 2019 due to suspected respiratory involvement were included. This suspicion was based on symptoms suggestive of hypoventilation (daytime sleepiness, poor sleep quality, morning headache), or diaphragmatic dysfunction (dyspnoea, orthopnoea, paradoxical breathing, use of accessory neck muscles), or abnormal pulmonary function tests, including vital capacity <80% of predicted value, maximal inspiratory pressure <60% of predicted value, or a >15% decrease in supine vital capacity [7,8]. Oxicapnography was recorded on average for 8h using a SenTec 92 device (Therwil, Switzerland). The combined Severinghaus-type TcPCO2 electrode and SpO2 sensor was calibrated according to the manufacturer's instructions before and after each measurement to adjust for calibration drift. The sensor was applied to the patient's earlobe at the usual bedtime and left for a mean recording time of 8h. Recording was performed at home or during an in-hospital sleep study. Stored data were analysed with a dedicated software (V Stats, Therwil, Switzerland). TcPCO2 trends were visually inspected by the same investigator (YC) to exclude any artefacts (absurd values or drift).
Oxicapnography was not performed in NMD patients (i) on oxygen therapy, (ii) pregnant, (iii) who had experienced a respiratory exacerbation within the previous two months, or (iv) with moderate-to-severe sleep apnea confirmed by poly(somno)graphy. ABG were taken at rest in room air. A venous blood sample was collected in the afternoon (4–6h before oxicapnography's beginning) to measure MVtCO2. According to the results of this exhaustive assessment, NIV was initiated according to current guidelines [2,9,10]. Our study was approved by the French Respiratory Society's ethics board (CEPRO 2020-009), and all participants provided written informed consent.
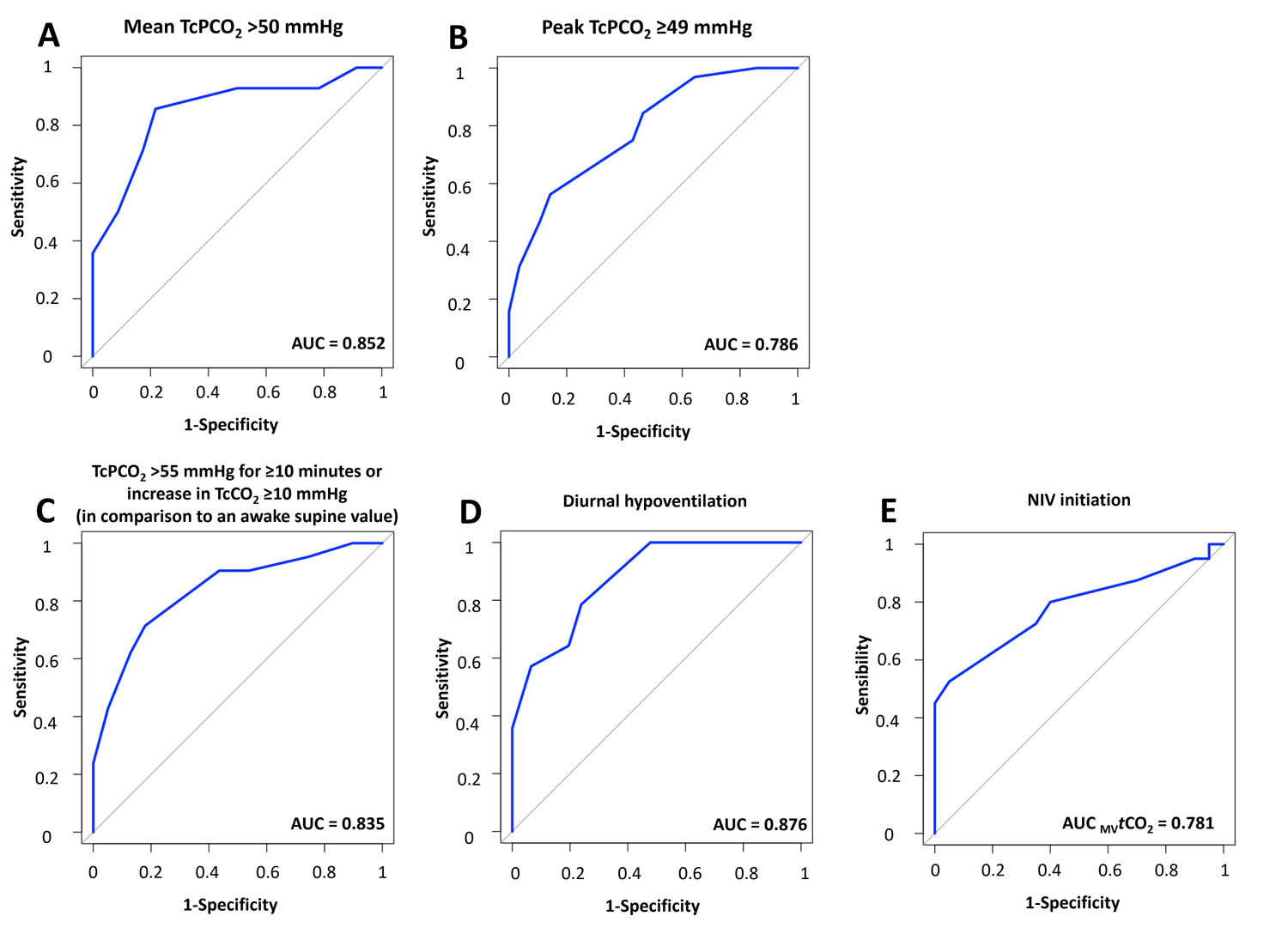
Diurnal hypoventilation was defined by PaCO2 >45mmHg. Nocturnal hypoventilation was assessed using three established TcPCO2-based criteria: (i) definition 1: mean TcPCO2 >50mmHg [11,12], (ii) definition 2: peak TcPCO2 ≥49mmHg [3], and (iii) definition 3: TcPCO2 >55mmHg for ≥10min or increase in TcCO2 ≥10mmHg (in comparison to an awake supine value) to a value exceeding 50mmHg for ≥10min [13].
Continuous variables are reported as medians with interquartile ranges (IQR) and compared using the Student t-test or Wilcoxon–Mann–Whitney test. Categorical variables were expressed as numbers (%) and compared using the Chi-squared or Fisher's exact test. A p-value <0.05 was considered statistically significant. Diagnostic performance was assessed through sensitivity, specificity, predictive values, and likelihood ratios. We used Youden's J statistic (sensitivity+specificity−1) [14] to identify the optimal diagnostic threshold, as it is unaffected by disease prevalence, a key consideration in our tertiary single-centre study. This index balances sensitivity and specificity, enabling us to choose a cut-point that maximizes sensitivity and negative predictive value. Prioritizing these metrics helps minimize false negatives (i.e., NMD patients with nocturnal hypoventilation with an unidentified need for NIV) and ensures early detection of NMD patients with nocturnal hypoventilation who may otherwise experience delayed NIV initiation or worsening respiratory function, posing a serious health risk. Fagan's nomogram was applied to estimate post-test probabilities [15]. Predictive performance of MVtCO2 was evaluated using the area under the receiver operating characteristic curve (AUC-ROC) [16], with 95% confidence intervals (CI) derived by bootstrap. All analyses were conducted using R version 4.3.1.
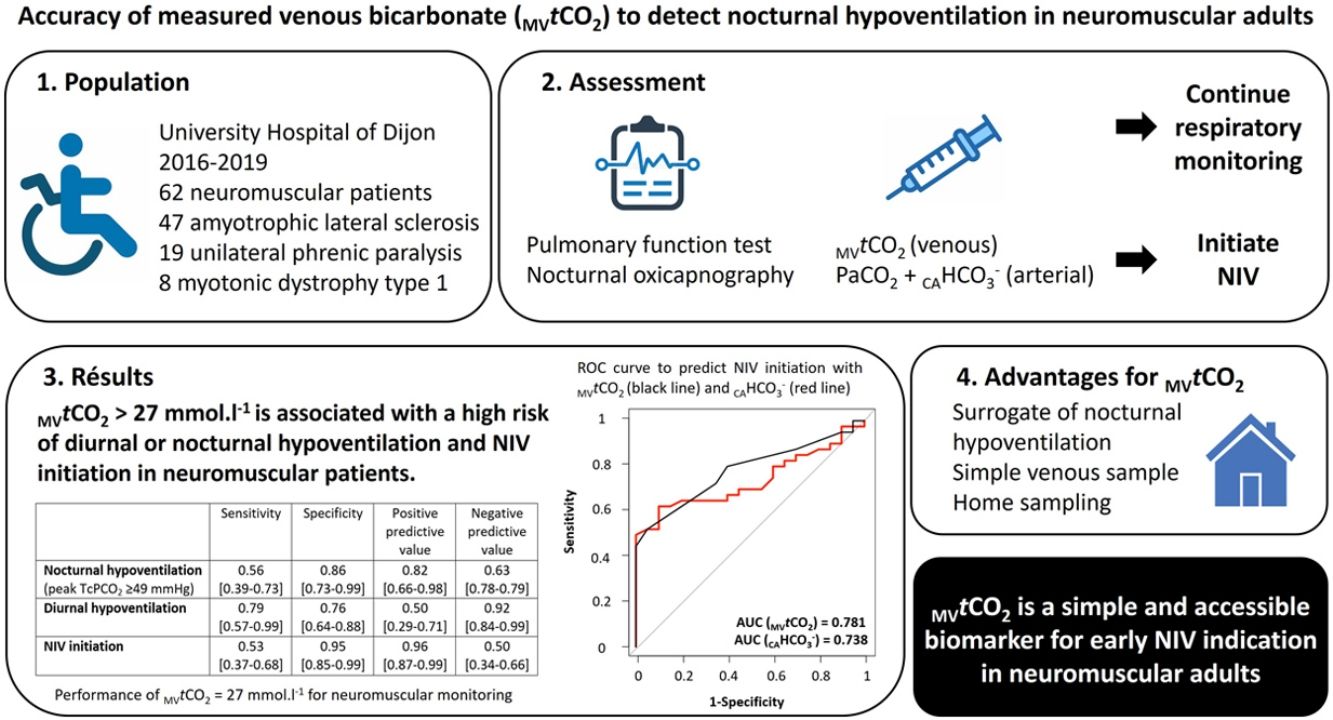
Sixty-two patients (35 men, 58.5 [44.7–67] years old, 22.4 [20.2–28.3]kg/m2) met inclusion criteria and 12 additional recordings were excluded due to technical issues. The most common NMD were amyotrophic lateral sclerosis (ALS) (47%), unilateral diaphragmatic palsy (19%) and myotonic dystrophy type 1 (8%) with restrictive pattern (median FVC: 71 [48–82]% of predicted value) and diaphragmatic weakness (SNIP: 39 [30.5–48.7]% of predicted value) (see supplemental data for detailed population characteristics). Overall, 68% of patients (n=42) initiated non-invasive ventilation (NIV). These patients had significantly higher PaCO2 (40.1 [37.9–43.5] vs. 43.9 [39.5–49.3]mmHg) and nocturnal peak TcPCO2 levels (49.9 [46.4–52.4] vs. 54.9 [47.9–61.1]mmHg), as well as elevated MVtCO2 (25 [24.7–27.2] vs. 28 [26–30]mmol/L), all p<0.01. Prevalence of hypoventilation varied depending on the definition used: for nocturnal hypoventilation, 22% with definition 1, 52% with definition 2, 34% with definition 3 and 22% for diurnal hypoventilation.
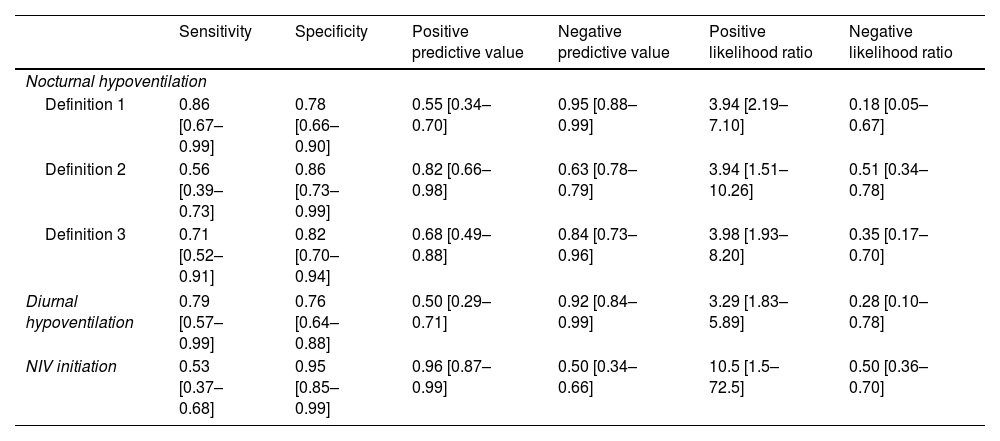
Table 1 show performance metrics for MVtCO2. The optimal threshold identified for predicting NIV need were 27mmol/L. Post-test probabilities derived from Fagan's nomogram indicated a 92% likelihood of requiring NIV when MVtCO2 exceeded 27mmol/L. Conversely, values below this threshold were associated with only a 33% probability of NIV need.
Diagnostic performance of MVtCO2=27mmol/L for neuromuscular patients’ screening.
| Sensitivity | Specificity | Positive predictive value | Negative predictive value | Positive likelihood ratio | Negative likelihood ratio | |
|---|---|---|---|---|---|---|
| Nocturnal hypoventilation | ||||||
| Definition 1 | 0.86 [0.67–0.99] | 0.78 [0.66–0.90] | 0.55 [0.34–0.70] | 0.95 [0.88–0.99] | 3.94 [2.19–7.10] | 0.18 [0.05–0.67] |
| Definition 2 | 0.56 [0.39–0.73] | 0.86 [0.73–0.99] | 0.82 [0.66–0.98] | 0.63 [0.78–0.79] | 3.94 [1.51–10.26] | 0.51 [0.34–0.78] |
| Definition 3 | 0.71 [0.52–0.91] | 0.82 [0.70–0.94] | 0.68 [0.49–0.88] | 0.84 [0.73–0.96] | 3.98 [1.93–8.20] | 0.35 [0.17–0.70] |
| Diurnal hypoventilation | 0.79 [0.57–0.99] | 0.76 [0.64–0.88] | 0.50 [0.29–0.71] | 0.92 [0.84–0.99] | 3.29 [1.83–5.89] | 0.28 [0.10–0.78] |
| NIV initiation | 0.53 [0.37–0.68] | 0.95 [0.85–0.99] | 0.96 [0.87–0.99] | 0.50 [0.34–0.66] | 10.5 [1.5–72.5] | 0.50 [0.36–0.70] |
Abbreviation. NIV: non-invasive ventilation.
Criteria for nocturnal hypoventilation are:
- Definition 1: mean TcPCO2 >50mmHg
- Definition 2: peak TcPCO2 ≥49mmHg
- Definition 3: TcPCO2 >55mmHg for ≥10min or increase in TcCO2 ≥10mmHg (in comparison to an awake supine value) to a value exceeding 50mmHg for ≥10min
Diurnal hypoventilation is defined by PaCO2 >45mmHg.
Data are presented as medians [first-third quartile].
MVtCO2 demonstrated strong predictive performance for diurnal (AUC 0.876 [95% CI: 0.783–0.968]) and nocturnal hypoventilation (definition 1: AUC 0.852 [95% CI: 0.723–0.980]; definition 2: AUC 0.786 [95% CI: 0.675–0.898]; definition 3: AUC 0.835 [95% CI: 0.783–0.968]) and NIV initiation (AUC 0.781 [95% CI: 0.669–0.893]) (see supplemental material for figure).
Our findings support the use of MVtCO2 as a practical screening biomarker for hypoventilation in NMD patients. A threshold of >27mmol/L was significantly associated with both nocturnal and diurnal hypoventilation and the need for NIV. MVtCO2 offers key advantages: it is non-invasive, more accessible, especially in low-income countries, and feasible even in severely disabled NMD patients. Venous sampling can also be performed at home, improving its potential as a widespread screening tool.
This threshold aligns with values proposed for OHS [8,17]. Due to the slower renal elimination of bicarbonate compared to CO2 clearance by the lungs, MVtCO2 serve as a surrogate marker of nocturnal CO2 retention. Residual daytime metabolic alkalosis indicates prior nocturnal hypoventilation [8]. Early identification and management of hypoventilation is crucial to prevent acute hypercapnic failure, particularly in patients with Duchenne muscular dystrophy, myotonic dystrophy type 1, and ALS [3,17,18]. Nonetheless, interpretation of elevated MVtCO2 must consider potential confounders. Several medications (e.g., antihypertensives, antidiabetics, proton pump inhibitors) may alter acid–base balance [19,20]. Only four patients were on such medications (3 for Duchenne cardiomyopathy), suggesting limited interference in our cohort. Most included patients, even ALS patients, were relatively young and had fewer comorbidities. Methodological issues also exist as CO2 dissolved in serum is volatile, leading to artificially low MVtCO2 values [21]. These potential discrepancies must be acknowledged when using bicarbonate thresholds.
The main limitation of our retrospective and monocentric study was the modest sample size, although reflective of the rarity of NMD. Our cohort was drawn from a tertiary center, with possible referral of more severe respiratory involvement explaining the fact that two-thirds of our patients required NIV. However, it is the same proportion that is observed in the studies by Orlikowski et al. [22] and Ward et al. [3] where respectively 42 and 90% of NMD patients with a peak TcPCO2 ≥49mmHg require NIV after 2 years of follow-up. This limits the generalizability of predictive values, which are prevalence-dependent [23]. For this reason, we emphasized likelihood ratios, which are more robust [24].
Moreover, because no consensus exists on the ideal strategy for defining nocturnal hypoventilation, we employed various recommended definitions to allow comparison with prior studies [3,12,13,21]. Transcutaneous capnography is increasingly regarded as the gold standard to assess nocturnal hypoventilation. However, despite recent technical improvements, TcPCO2 accuracy remains strongly dependent of appropriate handling and knowledge of the equipment and procedure and TcPCO2 interpretation mostly relies on expert opinions. Few data in NMD comparing TcPCO2 ability to predict relevant clinical outcomes suggest that the cut-off value (peak TcPCO2 ≥49mmHg) should be preferred. This reinforces the need for alternatives easier to interpret.
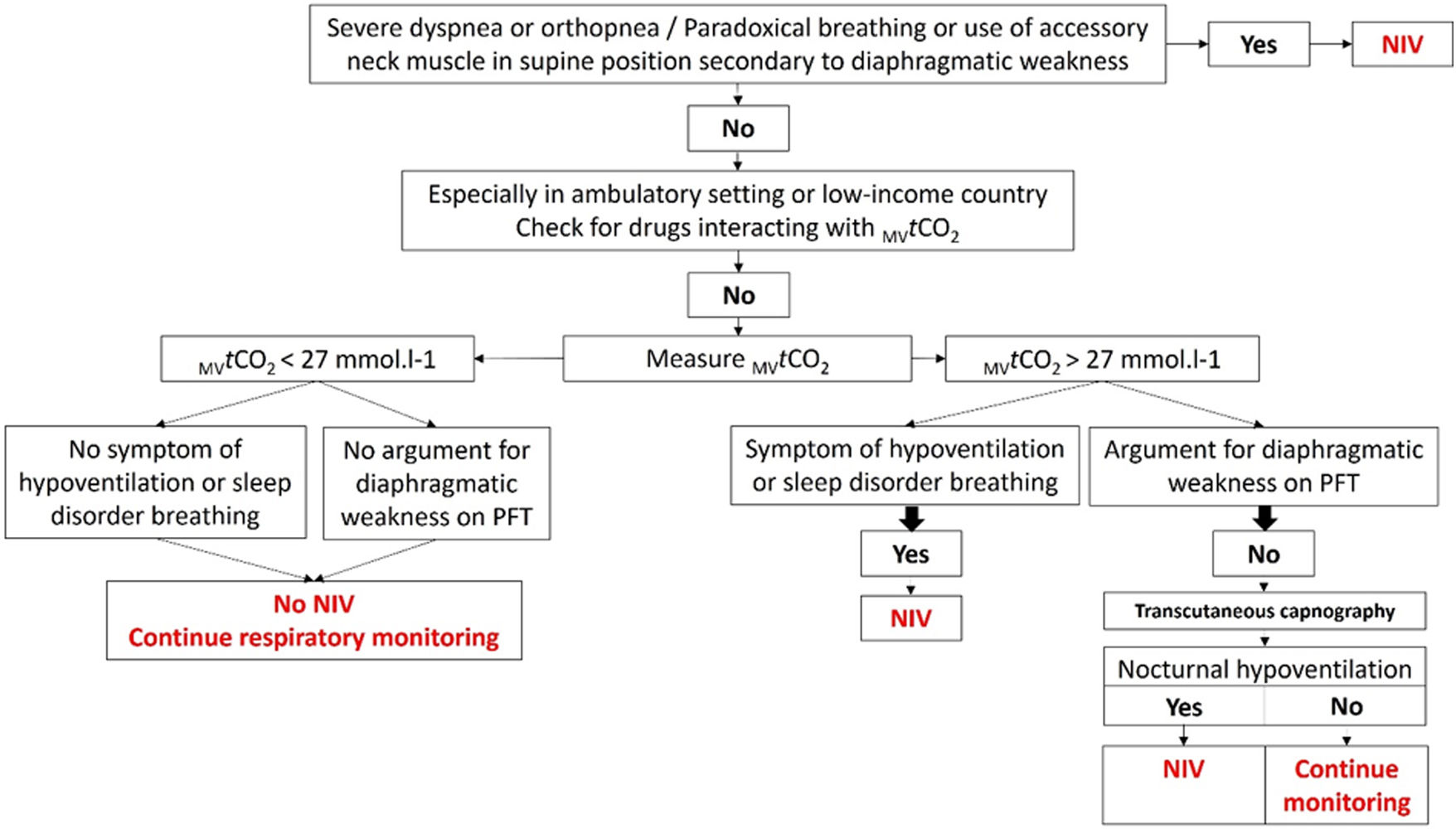
In conclusion, MVtCO2 >27mmol/L is a low-cost, accessible and easy marker that may help identify NMD patients at risk for hypoventilation and guide early NIV initiation. It could be incorporated into clinical screening algorithm (Fig. 1), but prospective multicentric validation is essential.
Algorithm including measured venous total carbon dioxide concentration (MVtCO2) to screen respiratory involvement in neuromuscular patients. Abbreviations: MVtCO2: measured venous total carbon dioxide concentration; NIV: non-invasive ventilation; PFT: pulmonary function test. Rythm for respiratory monitoring depends on the aetiology of NMD.
Data acquisition: YC, MG, CR, PM, DSC
Data analysis: YC, MG, CR, SA, ML
Manuscript drafting: MG, CR
Manuscript revision, editing and approval: all authors
Declaration of generative AI and AI-assisted technologies in the writing processNone declared.
FundingNone declared.
Conflict of interestMG declares personal fees from ResMed for participating to advisory board and from Asten Santé, Elivie SAS and Oxylis for speaking, lecture fees and travel reimbursement, all outside of this work.
CR declares personal fees from ResMed and Philips for participating to advisory board and from Asten Santé, Elivie SAS, ASDIA, Oxylis and ANTADIR for speaking, lecture fees and travel reimbursement, all outside of this work.
Other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors wish to thank Suzanne Rankin from the Dijon University Hospital for proofreading the manuscript and making the necessary corrections.