Chronic obstructive pulmonary disease (COPD) worsens the quality of life of the patients and causes morbidity and mortality. It represents a major burden for society and the healthcare systems due to the cost of medications and hospitalizations during exacerbations.1 In fact, exacerbating patients or “exacerbators”, i.e., those who present two or more moderate exacerbations (treated with antibiotics and/or systemic corticosteroids) or one exacerbation leading to hospitalisation in the previous year, account for the largest part of medical costs.2 Accurate implementation of evidence-based treatment recommendations results in improved healthcare outcomes and is a priority in modern healthcare systems.3
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) strategy provides recommendations for the prevention, diagnosis, treatment, and follow-up of patients with COPD. The GOLD initiative started in 1998 and the first report was released in 20014; since then, periodic updates have incorporated the growing evidence about the management of COPD. The 2023 Report has been updated with several important changes, including the addition of several new references and definitions, new figures and tables, and a new classification of the disease for initial treatment.5 Since 2011, the GOLD recommendations for initial pharmacologic treatment have been based on the intensity of symptoms and the frequency and severity of exacerbations, with several changes introduced with the different updates. The last update released in 2023 included a new classification of three subgroups “ABE”.5 Here, we present a proposal for a new graphical representation and an algorithm (or decision-tree) for initial treatment based on this new ABE classification.
The “GOLD ABE Assessment Tool”The new “GOLD ABE Assessment Tool” has replaced the former “ABCD Assessment Tool”, which was initially proposed in 2011. This tool was separated from spirometric grading in the 2017 Report6 and combined the parameters of exacerbation and symptom burden to categorise the patients into four groups: Group A included infrequent exacerbators with a low level of symptoms; Group B included patients with infrequent exacerbations but a high level of symptoms; Group C included patients with frequent or severe exacerbations and a low level of symptoms and; and Group D included patients with both frequent or severe exacerbations and a high level of symptoms. The symptomatic burden of COPD patients is evaluated with validated tools including the modified Medical Research Council (mMRC) dyspnoea scale7 and the COPD Assessment Test (CAT).8
The proposed initiation of pharmacological therapy for each group in the previous 2022 Report was with a bronchodilator for Group A, a long-acting bronchodilator (either a long-acting beta-2 agonist (LABA) or a long-acting anticholinergic agent (LAMA)) for Group B, a LAMA for Group C, and a LAMA or combination of LAMA plus LABA (if highly symptomatic) or combination of inhaled corticosteroid (ICS) plus LABA (if blood eosinophil count was ≥300cells/μL) for Group D.9
The updated “GOLD ABE Assessment Tool” has highlighted the importance of COPD exacerbations by dissociating the presence of the “exacerbator” phenotype from the symptomatic burden and grouping all exacerbators together irrespective of the presence and intensity of symptoms, thus giving priority to the presence of exacerbations over the respiratory symptoms. Consequently, Groups C and D are now merged into one group (Group E) and, therefore, patients are now separated into three groups: Group A includes patients with 0–1 moderate exacerbations in the previous year and mMRC 0–1, CAT <10; Group B with 0–1 moderate exacerbations in the previous year and mMRC ≥2 and/or CAT ≥10; and Group E includes “exacerbators”, i.e., patients with ≥2 moderate exacerbations or ≥1 leading to hospitalisation in the previous year, irrespective of the symptom burden. The proposed initiation of pharmacological therapy for each group in the 2023 Report was a bronchodilator for Group A, combination of LABA plus LAMA for Group B, and combination of LAMA plus LABA for Group E, with the exception of patients with more than 300 blood eosinophils/μL, who should be initiated with the triple combination of LAMA/LABA/ICS.5
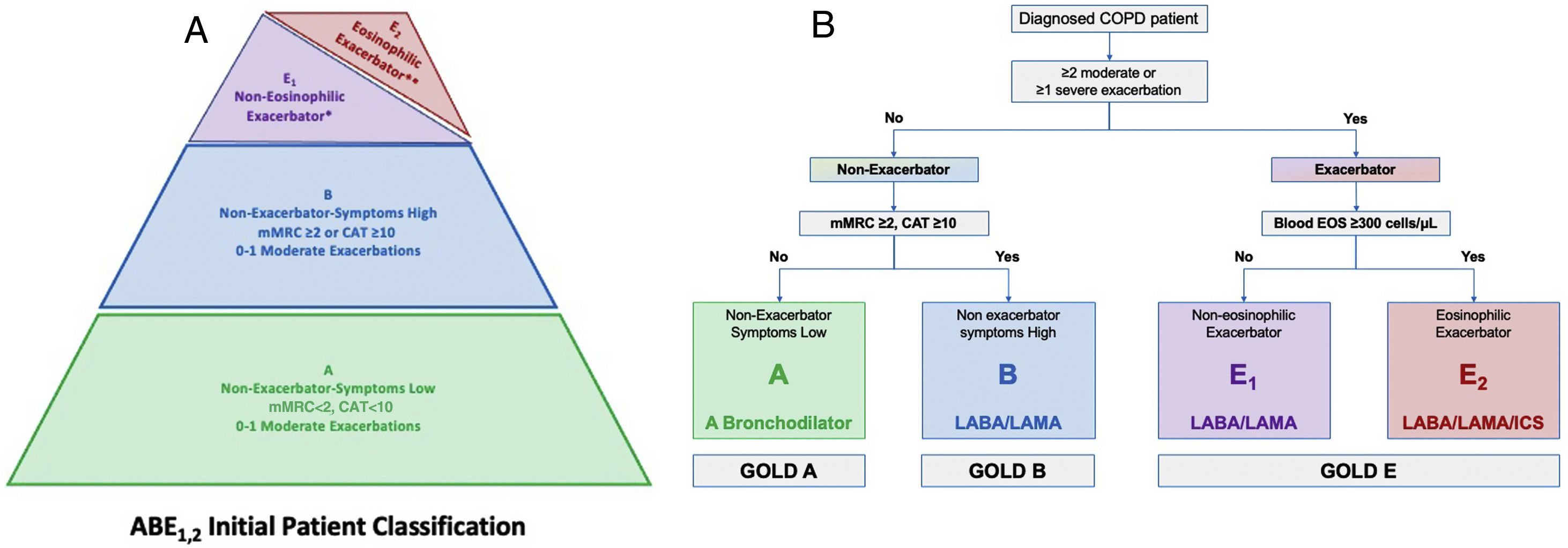
A Proposal for a New Figure and Algorithm for the “GOLD 2023 ABE Assessment Tool”The new classification of patients for initial pharmacologic therapy into three groups could be better represented by a pyramid-shaped scheme instead of the current scheme of the GOLD 2023 Report, in order to facilitate the visualisation and the understanding of the recommendations for initial pharmacotherapy in COPD patients and improving their implementation.
Tables, figures, and schemes are extensively used in education and research.10 Pyramids are particularly useful to show data in a more schematic and representative way, and for this reason are often preferred.11 The current scheme of the “GOLD ABE Assessment Tool” does not include any reference to the severity and frequency of any of the groups. In contrast, from an educational point of view, the proposed pyramid shows the data in a clearer and more informative way for the reader and, thus, the figure may be better understood and remembered by the clinician.
The basis of the pyramid represents the broader group of patients with milder symptoms and no history of frequent or severe exacerbations (the GOLD 2023 Group A), followed by the more symptomatic patients of the current GOLD 2023 Group B. The exacerbators, characterised by the presence of ≥2 moderate exacerbations or ≥1 exacerbation leading to hospitalisation in the previous year, represent a much smaller group, comprising about one third of the COPD patients and are placed in a smaller area at the top of the pyramid. These subjects are divided into “non-eosinophilic exacerbators” (i.e., those with <300 blood eosinophils per μL, proposed as Group E1), and “eosinophilic exacerbators” (i.e., those with ≥300 blood eosinophils per μL, proposed as Group E2).
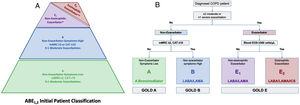
Based on this pyramid depiction of the ABE1,2 Groups, we propose a clinically relevant algorithm or decision-tree for the initial choice of therapy, in agreement with the GOLD 2023 recommendations. The decision-tree is initiated with the question of whether the patient can be classified as an exacerbator. If the answer is no, we need to investigate the level of symptoms to classify the patient into either Group A or B. If the patient fulfils the criteria for an exacerbator, they will be classified as Group E and the question will be whether their most recent blood eosinophil levels were above or below the threshold of 300cells/μL (subgroups E1 or E2). The final recommendations for treatment are a bronchodilator for Group A (non-exacerbators, symptoms low), whereas a LABA/LAMA combination is recommended in Group B (non-exacerbators, symptoms high). For the “non-eosinophilic exacerbators“of Group E1 the combination of LABA/LAMA is the first-line option, with triple therapy (LABA/LAMA/ICS) being the first choice in the “eosinophilic exacerbators” of Group E2.
Actually, although the new GOLD classification is called ABE it really includes four groups, because the recommendation for treatment of GOLD E is not unique and varies according to the blood eosinophil count, which, in fact, divides GOLD E into 2 subgroups. This is like the recent Spanish guidelines that classifies COPD patients into four groups to direct initial pharmacologic therapy: low risk – LAMA, high risk non-exacerbator – LABA/LAMA, high risk exacerbator non-eosinophilic – LABA/LAMA and high risk eosinophilic – LABA/ICS or LABA/LAMA/ICS.12
Developing an algorithm or decision-tree may help in guiding the initial selection of treatment by indicating the key elements that should be taken into account by the clinician. One more suggestion for the future would be to get rid of the letters (ABE) and substitute them for the description of the three different subgroups: “paucisymptomatic non-exacerbator”, “symptomatic non-exacerbator” and “exacerbator”. This would be even easier to remember and not subjected to misinterpretation about what the letters really mean by non-specialised clinicians.
ConclusionBased on the above, we propose a truncated pyramid shaped ABE1,2 scheme instead of the current figure as complementary to the GOLD 2023 “ABE Assessment Tool” and a simple algorithm to support the choice of initial pharmacotherapy options. We would like to stress that the final decision for the initial treatment options should be made by the attending physician in a personalised evidence-based manner and should reflect the clinical condition of the individual patient. Nevertheless, we strongly believe that the proposed pyramid ABE1.2 figure (Fig. 1) depicts the patient classification in a more representative way and complemented by our algorithm simplifies the initial choice of pharmacotherapy and may facilitate the wider implementation of the currently proposed GOLD ABE tool.
(A) The proposed truncated pyramid shaped ABE1.2 figure for the “GOLD ABE Assessment Tool”. (B) A proposed treatment algorithm or decision-tree for initial pharmacologic treatment of COPD based on the “GOLD ABE Assessment Tool”. CAT: COPD Assessment Test; EOS: eosinophils; mMRC: modified Medical Research Council. *Patients with ≥2 moderate exacerbations or ≥1 exacerbation leading to hospitalisation <300 blood eosinophils per μL. **Patients with ≥2 moderate exacerbations or ≥1 exacerbation leading to hospitalisation and ≥300 blood eosinophils per μL.
Marc Miravitlles has received speaker fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Menarini, Kamada, Takeda, Zambon, CSL Behring, Specialty Therapeutics, Janssen, Grifols and Novartis, consulting fees from AstraZeneca, Atriva Therapeutics, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, CSL Behring, Inhibrx, Ferrer, Menarini, Mereo Biopharma, Spin Therapeutics, Specialty Therapeutics, ONO Pharma, Palobiofarma SL, Takeda, Novartis, Novo Nordisk, Sanofi, Zambon and Grifols and research grants from Grifols.
Konstantinos Kostikas has received honoraria for presentations and/or consultancy fees from Alector Pharmaceuticals, AstraZeneca, Boehringer Ingelheim, CSL Behring, Chiesi, ELPEN, GILEAD, GSK, Menarini, Novartis, Pfizer, Sanofi, Specialty Therapeutics, WebMD; his department has received funding and/or grants from AstraZeneca, Boehringer Ingelheim, Chiesi, Innovis, ELPEN, GSK, Menarini, Novartis and NuvoAir; He is a member of the GOLD Assembly.
Nikoletta Bizymi has no conflicts of interest related to this paper.
Nikolaos Tzanakis has received speaker and/or consultancy fees from AstraZeneca, Boehringer Ingelheim, CSL Behring, Chiesi, ELPEN, GILEAD, GSK, Innovis, Menarini, Novartis, Pfizer, Sanofi, Specialty Therapeutics, Help and has received research grants from AstraZeneca, Boehringer Ingelheim, Chiesi, Innovis, GSK, Menarini, and Novartis.