Man-made mineral fibers are produced using inorganic materials and are widely used as thermal and acoustic insulation. These basically include continuous fiberglass filaments, glass wool (fiberglass insulation), stone wool, slag wool and refractory ceramic fibers. Likewise, in the last 2 decades nanoscale fibers have also been developed, among these being carbon nanotubes with their high electrical conductivity, mechanical resistance and thermal stability. Both man-made mineral fibers and carbon nanotubes have properties that make them inhalable and potentially harmful, which have led to studies to assess their pathogenicity. The aim of this review is to analyze the knowledge that currently exists about the ability of these fibers to produce respiratory diseases.
Las fibras minerales artificiales son fibras producidas por el hombre usando materia inorgánica que se emplean ampliamente como aislantes térmicos y acústicos. Incluyen básicamente el filamento continuo de fibra de vidrio, las lanas de vidrio, de roca y de escoria, y las fibras cerámicas refractarias. Así mismo, en las últimas 2 décadas también se han desarrollado fibras a nivel de nanoescala, entre las que destacan los nanotubos de carbono por su gran conductividad eléctrica, resistencia mecánica y estabilidad térmica. Tanto las fibras minerales artificiales como los nanotubos de carbono tienen propiedades que los hacen respirables y potencialmente nocivos, lo que ha conducido a la realización de estudios para valorar su patogenicidad. El objetivo de esta revisión es analizar los conocimientos que existen actualmente sobre la capacidad que tienen estas fibras de producir enfermedad respiratoria.
Fibers are long particles whose length is several times more than their diameter. The capability of a fiber to cause lung pathology is especially conditioned by the “3 Ds”: dimension, dosage and durability. As for dimension, inhalable fibers (meaning those that are able to reach the pulmonary parenchyma) are considered to be those having a diameter smaller than 3μm, a length greater than 5μm and a length/diameter ratio equal to or greater than 3. It is accepted that thicker fibers, although they could be inhaled, are usually retained in the upper respiratory tract, and the shorter ones could be phagocyted by the alveolar macrophages and eliminated. Dosage refers to the quantity of fibers that reach the pulmonary parenchyma and can cause pathology when their concentration surpasses the capability of the defense mechanisms to eliminate them. Durability, or biopersistence, is the time that a fiber can remain in the lungs. It is determined by the rate at which the fiber can be dissolved or broken down once deposited, which is related to its chemical composition. These 3 characteristics of fibers condition their capacity to reach, remain in and accumulate in the lungs,1 resulting in lung pathology.
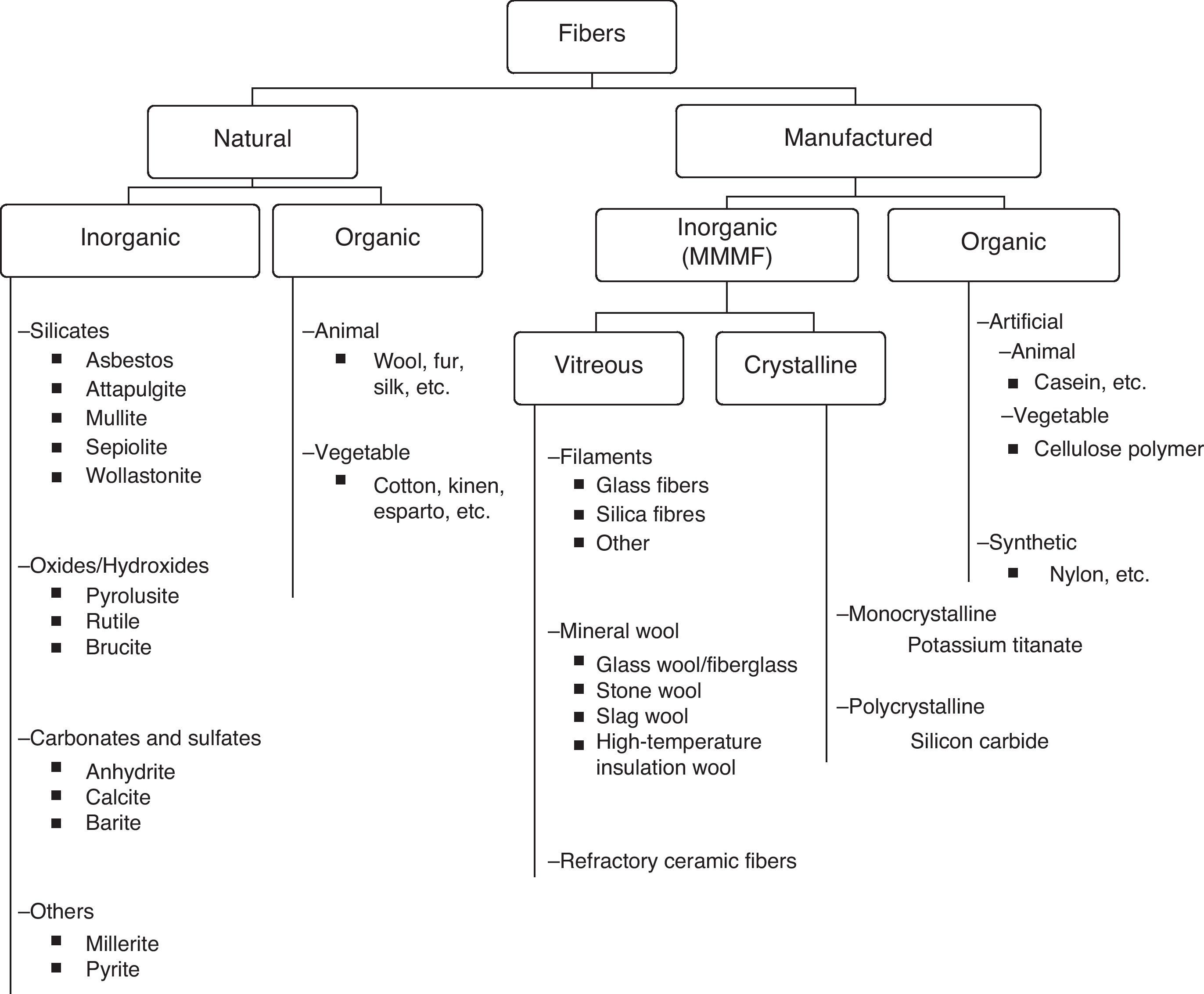
There are various types of fibers that can be classified in several ways.2–4 We propose for this review a classification according to origin and nature, shown in Table 1. First of all, the fibers are identified as either natural (as they are found in nature) or man-made (fabricated by humans). Both groups, natural and man-made, can be divided into organic or inorganic fibers. Organic natural fibers can be of animal (wool) or vegetable (cotton) origin. Within the inorganic natural fibers, there is a wide variety, and from this group asbestos stands out due to its ability to cause disease. Organic man-made fibers are those created by man using organic material. Within this group are artificial fibers, in which the raw material is natural but the process for obtaining the fiber is manufactured, like cellulose polymers, or synthetic, in which both the raw material and the process for obtaining the fiber are human fabrications, such as in the case of acrylic fibers or nylon.
Inorganic man-made fibers, produced by humans using inorganic material, can have a vitreous or crystalline structure. These are the most important man-made fibers due to the volume of their manufacturing and consumption, and they are generally known as man-made mineral fibers (MMMF). The most common MMMF have a vitreous structure (amorphous), and therefore MMMF are also generically known as synthetic vitreous fibers.
MMMF have some similarities with asbestos fibers, including the same aerodynamic properties. Nevertheless, while asbestos fibers generally tend to break up longitudinally, leading to long fibers that become thinner and thinner and can remain in the lungs over time, MMMF divide transversally, producing shorter and shorter segments that can be eliminated more effectively through the phagocytic system. In spite of these differences, due to the fact that there is so much information about the relationship between the inhalation of asbestos and the development of pleuropulmonary pathology, for years there have also been studies evaluating the possible toxicity of MMMF in the lungs and pleura. Furthermore, starting 2 decades ago, we now have the capability to manipulate matter on a nanoscale, leading to the appearance of nanoparticles, some of which have a fiber structure. The possibility that nanofibers could penetrate in the organism by inhalation and cause respiratory pathology has likewise awakened concern, and in recent years there have also been studies assessing their pathogenicity.
The objective of this review is to analyze the knowledge that exists today about MMMF and respiratory disease while updating a previous review that was published by this journal in 1996.5 First of all, the different types of MMMF are described, and then there is comment on studies in animals and in humans with each type. Since, as we have mentioned, the most common MMMF are vitreous and the studies in the literature have centered on these, in this review we will not deal with other MMMF types, and when we refer to “MMMF”, we refer to this type alone. Afterwards, and due to growing interest, we will specifically discuss nanofibers and the possibility of their causing respiratory pathology. The last part of this review provides the recommendations of national and international organisms for the use of MMMF and nanofibers.
Man-Made Mineral FibersMMMF are produced by melting raw material and giving it the desired shape by cooling it quickly. The most commonly used raw material is composed of silicates and varying quantities of inorganic oxides.
MMMF are typically classified into 3 types: continuous filament glass, mineral wool and refractory ceramic fibers (RCF).
- •
Continuous filament glass fibers are more or less rectilinear, with uniform diameters, and are typically thicker than mineral wool fibers. As they become fragmented, the fibers break up into shorter fibers, but due to their thickness (between 3.5 and 25.0μm), they are not considered inhalable. They began to be manufactured at the beginning of the 20th century and they are basically used to reinforce materials used in insulation, electronics and construction industries.
- •
Mineral wools are masses of interlocking, disorganized fibers with variable lengths and diameters, some of which may be inhalable. Mineral wools are classically divided into 3 types: fiberglass, stone wool and slag wool. Stone and slag wools were the first to be manufactured in the mid-nineteenth century, with a peak production in the mid-twentieth century, when glass wool (fiberglass insulation) gained in importance. They are basically used for thermal and acoustic insulation, typically in buildings, vehicles and appliances, as well as for inflammable materials and flame-retardant protection.
Fiberglass microfibers, which are glass wool fibers with a diameter smaller than 1μm, merit special attention. They are used in high-tech products, as high-efficiency air filters, or in aerospace insulation.
Since 1990, a new family of mineral wools has been developed, known as high-temperature insulation wool, which is made of alkaline-earth silicates. They are less biopersistent than RCF, have similar physical properties and can substitute these in some applications.
- •
Refractory ceramic fibers are a mix of aluminum, silica and other refractory oxides. Their fibers have a diameter of 1.2–3.5μm, and their longitude is variable. They began to be commercialized between 1950 and 1960, and they are relatively new compared with other MMMF. They have several possible applications, but they are basically used as thermal insulation for high-temperature requirements, mainly at the industrial level.
Animal studies that evaluate the potential effects of MMMF on the respiratory tract have been done in rodents, particularly rats and hamsters. The latter are currently considered either imperfect or even inadequate for evaluating the toxicity of fibers in humans due to their lung architecture and ultrastructure, the excessive sensitivity of their pleura and the difficulty for developing lung cancer when exposed to mineral dust and biopersistent fibers.1 The types of administration used have been intrapleural, intraperitoneal, inhalation and intratracheal instillation. There is a debate about the suitability of using the intrapleural and intraperitoneal pathway for evaluating the carcinogenic risk of inhaled fibers, since these methods of administration are different from the standard entry and circumvents the natural defense mechanisms of the organism. Intracavitary instillation studies of different types of MMMF have often shown tumor induction, mostly mesotheliomas.6,7 In fact, it has been observed that, at sufficient doses, all mineral fibers can cause carcinogenesis.8 However, the high pathogenicity seen in this administration type has not been demonstrated in epidemiologic studies in persons or in animals with inhaled administration. For these reasons, today it is largely considered that the results of long-term, well-designed studies with inhaled administration are the best for predicting the effects on human health.9 As for the mentioned studies with inhaled administration, they initially presented important limitations due to the technology of the era, such as the use of short fibers. Towards the end of the 1980s, a new generation of these studies, initiated by the Research and Consulting Company (RCC) with a much greater control over all working conditions, provided much more reliable results. Below, we comment on the most significant of these, according to MMMF type.
Glass Wool/Fiberglass InsulationSince the fibers that come from continuous filament glass are considered non-inhalable due to their size, studies have basically centered on fiberglass. Initially, the inhalation of glass wool fibers by rats showed no evidence of carcinogenesis.7,10–15 More recently, RCC researchers16,17 confirmed that the inhalation of 2 different types of fiberglass in rats for 2 years did not cause tumors or fibrosis. Two types of fiberglass microfibers deserve special mention as they are more biopersistent: 475 and E. In an inhalation study in hamsters, it was observed that fiberglass 475 did not induce lung tumors18 but did induce lung fibrosis and one case of mesothelioma. Cullen et al.,19 however, reported lung cancer as well as mesotheliomas in rats who were exposed to inhaled fiberglass E.
Stone Wool and Slag WoolSeveral studies that had evaluated the possibility of developing fibrosis or cancer through the chronic inhalation of these types of mineral wools obtained negative results.7,12,14 In addition, a more recent study by the RCC, in which rats were exposed to nasal inhalation of a type of stone wool and another type of slag wool, also showed no evidence of the development of neoplasms.20 In the case of stone wool, however, the exposed rats developed minimal fibrosis.
High-Temperature Insulation WoolTwo less biopersistent fibers that have been developed recently (alkaline earth silicate [X-607] and a wool with a low silica and high aluminum content [HT]) have been used in long-term inhalation studies in rats with no significant increase in the incidence of pleuropulmonary tumors.21 There has also been no observed tumor development after its intraperitoneal administration.22
Refractory Ceramic FibersInitial studies with chronic inhalation described the appearance of fibrosis and tumors in rodents, although the results were not considered reliable.23,24 Later RCC studies based on the nasal inhalation of RCF also showed evidence of developing fibrosis and tumors.25–27 However, when these studies were later analyzed, it was believed that there had been a phenomenon of lung overload.28 Rats seem susceptible to this phenomenon, which means that reaching a high concentration of particles and fibers may impede lung clearance mechanisms, causing inflammation and tumors much more easily. Said concentration would be several times higher than the possible human exposure and would not be representative of that found in the workplace. Thus, while in humans occupational exposure is approximately 0.2fiber/cm3 of air,29 inhalation studies in rats have used exposures that go from 100 to more than 1000fibers/cm3. More studies are considered necessary with exposures to lower particle and fiber concentrations in animal experiments in order to properly assess these results.30
Man-Made Mineral Fiber Studies in Cell CulturesAlthough in vitro studies are not considered appropriate for evaluating the toxicity of fibers,1 the International Agency for Research on Cancer (IARC)31 considered in 2006 that, overall, this type of studies are useful for discriminating between primary and secondary genotoxicity. They also may help detect potential adverse effects of new fibers and identify their mechanisms of action. For example, studies in cell cultures have shown that fiber toxicity is directly related with their length, as well as the fact that MMMF induce neoplastic transformation32,33 and genetic damage.34,35
Epidemiologic Studies of Man-Made Mineral Fibers in HumansStudies About Respiratory Tract CancerAsbestos fibers can produce 2 types of neoplasms in humans: malignant mesothelioma and lung cancer (LC). Due to the similar forms of the different MMMF and asbestos fibers, epidemiologic studies in populations exposed to MMMF have been especially based on the study of these 2 types of neoplasms; when we refer to them in this document, we will use the term respiratory tract cancer.
There are 2 large cohort studies that have been completed, one in the Unites States and one in Europe, and case–control studies from these cohorts. All these studies initially provide most of the epidemiologic evidence about the potential risk for respiratory tract cancer and other tumors also associated with occupational exposure to continuous fiberglass filament and mineral wools. The cohort study in the US was begun in the 1970s and initially included 16661 workers at 17 plants that produced fiberglass and mineral wool. The results were evaluated from the follow-up until 198536; later, the cohort was extended, and in the end 32110 workers were evaluated with a follow-up until 1992. The European cohort included 25000 workers from 13 plants that manufactured fiberglass and mineral wool, with a follow-up until 198237 that was then extended until 1990. The results of these studies are commented below according to the type of MMMF. The results of other study cohorts are also mentioned.
Continuous Fiberglass Filament- •
Cohort from the United States38,39—Two of the plants only produced this type of fiberglass and the analysis did not show evidence of increased mortality due to respiratory tract cancer compared with local cancer rates.
- •
European cohort40—No evidence was found of increased LC in workers exposed to continuous fiberglass filaments, although the population examined in this cohort was small.
- •
Other studies41,42—Another 2 cohort studies also showed no evidence for increased respiratory tract cancer risk.
- •
Cohort from the United States38,39—A statistically significant increase of 6% was observed in the mortality due to LC using local cancer rates. However, the incidence was greater among the workers who had been exposed less than 5 years. When we analyzed those who had been exposed for longer periods, this excess diminished and was no longer statistically significant. Mortality was also not related with the duration of the exposure or with the accumulated exposure to inhalable fiberglass. Moreover, when adjusted for smoking, based on a sample of male workers from the cohort, it was determined that tobacco smoke could be responsible for this higher risk for LC seen in the workers. There was no confirmed increase in the incidence of mesotheliomas or of any other non-respiratory neoplasms.
- •
Case–control study from the US cohort39,43—None of the factors, such as the duration of the exposure, the mean exposure intensity, and the onset time of fiberglass exposure, were related with an increased risk for respiratory tract cancer. Among the confounding factors in this study, smoking was a statistically significant predictor for LC risk.
- •
European cohort40,44—In the fiberglass workers, a certain excess of LC was found, which was clearly reduced by adjusting for the levels of national mortality. There was no correlation with the exposure time or onset. In this cohort, one case of death due to mesothelioma was observed. Nevertheless, in 2 of the factories, there was also documented exposure to asbestos45; in the remainder, there is no information available about other possible occupational exposures to other agents at the workplace or of the workers’ tobacco habit. The case–control study also showed no evidence of a relationship between LC and fiber diameter, duration of the exposure or time since the start of exposure.46 Nonetheless, it should be kept in mind that the exposure levels were low and the number of cases was small.
The studies usually analyze these 2 types of mineral wool in conjunction:
- •
Cohort from the United States38,47—The study showed evidence of a risk for respiratory tract cancer compared with national and local cancer rates. However, no association was confirmed with the duration of the exposure or the time transpired from the initial exposure. When adjusted for smoking, this risk disappeared.
- •
US case–control study47—No evidence was seen of an association between respiratory tract cancer and accumulated exposure to breathable fibers, both without as well as with adjustment for possible confounding factors like smoking or exposure to other occupational agents.
- •
European cohort—Simonato et al.37 reported an excess of LC among stone wool and slag wool workers exposed during a period prior to the introduction of proper safety measures for the suppression of dust. In 2 out of the 7 factories studied, the workers had possible exposure to asbestos, and it was precisely in these 2 factories where 70% of deaths due to LC occurred. The authors concluded that the results were not sufficient to be able to attribute the increase in LC to stone wool or slag wool, although they could not rule out that these may have contributed to increased risk.
- •
Other studies—Kjaerheim et al.48 carried out a case–control study that took into account occupational exposure to other products and smoking history. The results did not show evidence of increased risk for LC, pleural mesothelioma or any other type of tumor. In 2002, Berrigan et al.49 also carried out a meta-analysis of cohort studies in workers and they found a significant increase in the risk for respiratory tract cancer death among workers exposed to stone and glass wool, although they considered that this increase could be either in part or totally due to smoking. In 2009, Lipwort et al.50 reviewed the epidemiological studies that analyzed respiratory tract cancer risk in workers exposed to fiberglass and stone wool, and they carried out a meta-analysis. The meta-analysis, based on cohort studies of MMMF production workers and community-based case–control studies, showed evidence of a discrete increase for LC risk. However, there are several factors that lead one to believe that this discrete increase was not due to a causal relationship of MMMF. There was not a causal dose-risk relationship in most studies that assessed the levels of exposure to MMMF and LC risk. The discrete increase was not seen in studies of ex-workers or in workers of MMMF applications, despite the comparable exposure levels. Finally, there were possible confounding factors, such as smoking or simultaneous exposure to asbestos. For this reason, the authors considered that there was not sufficient evidence to be able to attribute a causal relationship between glass and stone wool and LC. In the case of pleural mesothelioma, there is a single study that has reported a slight increase after adjusting for asbestos exposure.51 Nonetheless, the lack of an increased risk for this disease in the most powerful cohort studies52 reduces its credibility.
Chiazze et al.41 carried out a case–control study in men with LC from a cohort of 2933 workers at a continuous fiberglass filament factory. They assessed the exposure to fiberglass, asbestos and RCF, among others. The risk for LC was lower in those exposed to RCF compared with the controls. In 2003, LeMasters et al.53 published the results of a cohort study with 942 workers exposed to RCF between 1952 and 1997. The mortality results related with respiratory diseases were negative. When they compared the survival rates related with the accumulated exposure to RCF, no correlation was found. There were no cases of mesothelioma. This study, however, had several limitations. On one hand, the young age of the cohort: the average age of the workers at the end of the follow-up was 51, and the average follow-up period was 21 years. On the other hand is the small sample size, as the follow-up group was made up of less than 100 workers.
Studies About Other Respiratory DiseasesContinuous Fiberglass Filament and Mineral WoolsOne of the most important studies included 1089 workers from 5 fiberglass factories and 2 mineral wool factories in the United States.54 Each worker completed a respiratory questionnaire and respiratory function tests, and had chest radiography. In the study population, no respiratory symptoms or functional repercussions were found, but there was a low incidence of small non-specific pulmonary opacities seen on radiography. Nevertheless, when the study was extended to include more than 1400 workers and 300 controls, the researchers concluded that they had not found signs of clinical, functional or radiological affectation.55 There are isolated cases that have been published of lung fibrosis in people exposed to fiberglass. Takahashi et al.56 described the case of a carpenter exposed for more than 40 years in a glass wool industry who presented nodular opacities on chest radiology that was predominantly bibasilar, with interstitial fibrosis and detection of fiberglass in the transbronchial biopsy. Guber et al.57 published the case of a male with pulmonary fibrosis, whose transbronchial biopsy and induced sputum demonstrated fibers that were compatible with fiberglass. In this case, the patient had been the driver of a bus whose ceiling, which was covered in fiberglass insulation, was deteriorating and fibers were dispersed throughout the interior of the vehicle. In both cases, it was suggested that the fiberglass fibers could have been involved in the development of the disease. Drent et al.,58,59 on the other hand, have reported 14 cases of granulomatous lung disease in subjects who were exposed to continuous filaments and mineral wools. The clinical, radiological and bronchoalveolar lavage characteristics were identical to those of sarcoidosis, and in the pathology study granulomas were seen. In 6 of the cases, MMMF were detected by electron microscope. The authors suggested that, in susceptible persons, the exposure to these MMMF could trigger granulomatous disease similar to sarcoidosis, such as that also produced by the inhalation of dust from certain metals, like beryllium or aluminum. One study in workers at a factory of glass microfibers verified an increase in asthma symptoms related with exposure, although it was not statistically significant. The authors concluded that the sample was not able to clarify whether glass microfibers could cause occupational asthma.60 It should also be mentioned that, during the manufacturing process at the said factory, sensitizing agents such as formaldehyde were used.
Refractory Ceramic FibersSeveral studies have assessed respiratory function and radiology in RCF workers. Some of these have confirmed a decline in FVC and/or FEV1 in workers who smoke or were ex-smokers.61,62 Given the fact that the decline in FEV1 was limited to smokers, it has been postulated that the fibers could contribute to the effect of smoking on airflow.63 In 2011, McKay et al.64 published the results from the follow-up of 1396 workers and ex-workers for a maximum of 17 years and they found no evidence of a consistent decline in lung function. In another follow-up study, no reduction was observed in carbon monoxide diffusion capacity (DLCO) related with exposure.65 As for radiological studies, Lockey et al.66 completed a longitudinal, radiological follow-up study in 1008 workers who worked in RCF manufacturing. They found pleural changes in 2.7% of the workers, mainly pleural plaques, and they observed an association between the level of accumulated exposure and the appearance of pleural plaques. The European study by Cowie et al.65 had also confirmed the appearance of pleural plaque in these workers, but their finding was not related to the intensity of exposure. In neither of the 2 studies was evidence shown for parenchymal lung disease. In spite of this, in most of the cohort studies in MMMF factory workers, exposure levels were estimated to be low; therefore, it is considered that the epidemiological studies may not have detected cases of pulmonary fibrosis for this reason.
NanofibersNanoparticles are defined as particles with a dimension of 100 nanometers or less. There is an ample diversity of nanoparticles derived from inorganic non-metallic elements such as carbon, silica or boron; inorganic metallic elements such as gold, silver or other metals; and organic or biological elements such as liposomes or virus. Using inorganic non-metallic elements, nanotubes are constituted, and although they are made of diverse materials, usually the term “nanotubes” is used for those made of carbon, as they are the most widely developed and used due to their physical and chemical characteristics. Carbon nanotubes (CNT) have great electrical conductivity and mechanical resistance (they are considered the most resistant fiber that can currently be manufactured) and are enormously stable thermally. These unique characteristics make them very useful for several applications, among these electronics, industrial engineering and medicine, among other fields. Structurally, CNT can be described as graphite sheets that are rolled into cylinders with one or more layers of thickness. They go from one to several nanometers wide and several micros in length, and this width/length ratio make them structurally similar to other fibers, like asbestos. The possibility that the inhalation of CNT could cause pathology similar to what is caused by asbestos has raised concern, and in recent years its pathogenicity has been researched.
Carbon Nanotubes and Diffuse Interstitial Lung DiseaseWhile the studies with intratracheal and pharyngeal instillation of CNT have shown evidence of inflammation and pulmonary fibrosis,67–71 those that are done by inhalation have demonstrated alveolar lipoproteinosis72 and systemic immunosuppression.73 Most likely, the quantity and characteristics of CNT deposited in the lungs according to the method of administration would explain these differences.74 Studies with intratracheal or inhaled administration of CNT in animals have also verified the appearance of granulomas in the lung parenchyma and mediastinal lymph nodes.67,71,72,75
Carbon Nanotubes and Pleural DiseaseSeveral experimental studies have assessed the risk for developing mesothelioma after exposure to CNT. Inhalation studies in rats have shown evidence of the development of pleural fibrosis.76 With intraperitoneal administration, several studies have shown the appearance of inflammation and granulomas in the pleura,77,78 mainly when longer CNT have been administered (between 20 and 100μm).77 Until now, only one study with intraperitoneal administration79 and another with intrascrotal administration80 have shown evidence of the development of mesothelioma. In the first, micrometric CNT were administered in a mouse species that was genetically modified with predisposition for developing cancer. The authors considered that the capability of CNT of this length to induce mesothelioma had been demonstrated, but they affirmed that this result could not be extrapolated to nanometric-sized CNT. This study, however, has been criticized for the type of mouse used, the inappropriate exposure method, the high dosage of exposure, the underestimation of the number of CNT and for the poorly illustrated histology.81,82 In the intrascrotal administration study, the same type of CNT was used with the same doses, and the same method was followed as in the intraperitoneal administration, although in this case the mice used were not genetically modified. Later, Muller et al.83 carried out another study with intraperitoneal administration in mice in which the CNT were shorter than 1μm on average, with no evidence of the development of mesothelioma. Given the results of these studies and the known fact that the ability of asbestos fibers to cause fibrosis and cancer depends on their longitude, it is easy to think that CNT could behave in a similar manner.
Carbon Nanotubes and Allergic AsthmaThe intratracheal instillation of CNT in mice has caused the appearance of allergic responses through the activation of B cells and the production of IgE in the absence of previous allergic asthma.84 Furthermore, there are also other studies in mice that confirm that CNT exacerbate airway inflammation in mice who had previously been induced with allergic asthma.85,86 Thus, these studies lead us to believe that CNT could act as allergens themselves and can also exacerbate previous asthma. Although this has been observed in rats, it is not known whether CNT can trigger or exacerbate bronchial asthma in humans.
Carbon Nanotubes and GenotoxicityThe potential genotoxicity of CNT is currently uncertain because papers that have tried to assess it have shown contradictory results and suggest different possible mechanisms of action.87 In addition, it is unknown whether the standard tests that are used, which are designed to evaluate soluble chemicals, are equally effective for testing the genotoxicity of nanomaterial.
RecommendationsIn 2002, the IARC considered that there was not sufficient evidence of the carcinogenicity in humans of continuous fiberglass filament, mineral wools and RCF.4 Nonetheless, it underlined that the epidemiological studies had limitations that should be taken into consideration when interpreting the results, like the possibility of a poor assessment of the quantity of exposure to fibers, the difficulty to assess the risks of the workers exposed to the more durable fibers, the difficulty to adjust for confounding factors like smoking or exposure to asbestos, or the limitation of most workers being exposed to low levels of fibers. It was also emphasized that there were no data about the repercussions of MMMF exposure in workers who do not manufacture but instead use these fibers or work in their removal (e.g. in construction), and who may experience exposures that are occasionally higher, although on a more intermittent basis. For the IARC, however, in animal experimentation there is enough evidence for a specific group of fiberglass (such as E and 475), as well as RCF, to be considered carcinogenic. For fiberglass insulation, stone wool, slag wool and some more biopersistent fibers, like H fiber, the evidence of carcinogenicity in animal experimentation is limited, while for continuous fiberglass filament and for less biopersistent fibers there is not sufficient evidence. Thus, the IARC classifies fiberglass like E and 475, as well as RCF, as possible carcinogens in humans (group 2B); meanwhile, continuous fiberglass filament and glass, stone and slag wool are considered non-classifiable with regard to their carcinogenicity in humans (group 3).
In Spain, the National Institute for Occupational Safety and Hygiene (in Spanish, INSHT) has published guidelines for the determination of asbestos fibers and other fibers in the air using phase contrast microscopy.88 Since 2000, the guidelines incorporate the permissible exposure limit (PEL) for fibers other than asbestos in the list of limits for occupational exposure of chemical agents.89 For RCF and special-use fibers, the limit for occupational exposure is set at 0.5fiber/cm3, and for fiberglass and mineral wool, 1fiber/cm3. Although the exposure to MMMF during its production, use and elimination is believed to have been higher in the past, current mean exposure levels are generally less than 0.5inhalable fibers/cm3 (500000inhalable fibers/m3) in an average of 8 work hours. Higher levels have been measured, however, in the production of special-use fiberglass and RCF, and in the installation and removal of certain insulations. The concentrations of MMMF in the indoor and outdoor air in non-work settings are much lower.90–92 In addition to controlling PEL for the protection of workers, there are also recommendations about safety in the use of MMMF, such as those published by the International Labour Organization.93
With regards to nanofibers, conventional systems for detecting fibers, such as those used for asbestos or MMMF, have not been demonstrated to be practical and there is no international consensus about techniques for measuring nanoparticles in the workplace. Furthermore, we currently do not know the levels at which these nanofibers have adverse health effects, and there are therefore no specific exposure limits. For small-scale production situations, a simplified method has been developed for the qualitative assessment of risk of exposure to nanoparticles that allow decisions to be made about the necessary preventive measures.94 INSHT has also published technical reports with recommendations for the control of exposure to nanoparticles, using this simplified methodology.95,96
ConclusionsAlthough in experimental studies in rodents the intraperitoneal administration of high concentrations of mineral wools has caused the development of mesotheliomas, several studies with inhaled administration have not confirmed the appearance of neoplasms. Epidemiological studies in workers exposed to continuous fiberglass filament and mineral wool have also not been able to provide consistent evidence of association between the exposure to these fibers and risk for lung cancer or mesothelioma. One exception is about more biopersistent glass fibers, like E and 475, which have demonstrated in studies of intrapleural and inhaled administration the development of mesotheliomas and, in the case of E, lung cancer. For these reasons, continuous fiberglass filament and mineral wools are considered to have a non-classifiable carcinogenicity in humans, while more biopersistent fiberglass, like E and 475, are considered possible carcinogens.
As for the development of pulmonary fibrosis, animal experimentation studies using inhalation of glass wool have not confirmed its appearance, although minimal fibrosis was seen when stone wool was used. Isolated cases of lung fibrosis have been reported in workers exposed to fiberglass, and there have been reported cases of granulomatous disease similar to sarcoidosis in workers exposed to different mineral wools.
Regarding RCF, the information in the literature is more confusing. Experimentation studies have shown the induction of mesotheliomas after intraperitoneal administration, as well as fibrosis and tumors after the inhalation of high concentrations of RCF, but the interpretation of the results is difficult due to the lung overload phenomenon. Epidemiological studies have observed the appearance of pleural plaques in exposed worker, but no other type of diseases, such as interstitial fibrosis, lung cancer or mesothelioma, has been detected. Nevertheless, the results of epidemiologic studies with RCF are considered limited, and given the fact that asbestos induces pleural plaques and also pleural mesothelioma, there is concern about the possibility of RCF causing this type of tumor. For these reasons, RCF are currently considered possible carcinogens for humans. In recent years, programs have been developed that control exposure to RCF, but situations may arise with exposure to high concentrations that are less controlled, such as in demolition, where proper respiratory protection and adequate worker follow-up are both necessary.
In mice, CNT have been demonstrated to cause inflammatory, immune, fibrogenic and granulomatous responses according to the quantity of nanofibers and the administration pathway. It seems that inhaled CNT do not cause lung fibrosis, unlike when using intratracheal or pharyngeal administration, although they do cause pleural fibrosis. Several studies have evaluated the possibility that CNT could induce mesotheliomas, and only in 2 has there been evidence of this possibility, although only after the administration of characteristically long CNT (one with intraperitoneal and the other with scrotal administration). More studies are therefore needed in order to assess the risk of CNT for developing fibrosis and lung neoplasms. Until the biological and carcinogenic properties of nanoparticles are completely determined, measures should be taken to adequately control the exposure to these fiber nanomaterials, especially those that are longer and biopersistent.
Conflict of InterestsThe authors declare having no conflict of interests.
We would like to thank Dr. Ramon Magarolas and Mr. Xavier Guardino for the scientific review of this article.
Please cite this article as: Costa R, Orriols R. Fibras minerales artificiales y aparato respiratorio. Arch Bronconeumol. 2012;48:460–8.