Exposure to biomass smoke is a risk factor for chronic obstructive pulmonary disease (COPD). It is unknown whether COPD caused by biomass smoke has different characteristics to COPD caused by tobacco smoke.
ObjectiveTo determine clinical differences between these two types of the disease.
MethodsRetrospective observational study of 499 patients with a diagnosis of COPD due to biomass or tobacco smoke. The clinical variables of both groups were compared.
ResultsThere were 122 subjects (24.4%) in the biomass smoke group and 377 (75.5%) in the tobacco smoke group. In the tobacco group, the percentage of males was higher (91.2% vs 41.8%, P<.0001) and the age was lower (70.6 vs 76.2 years, P<.0001). Body mass index and FEV1% values were higher in the biomass group (29.4±5.7 vs 28.0±5.1, P=.01, and 55.6±15.6 vs 47.1±17.1, P<.0001, respectively). The mixed COPD-asthma phenotype was more common in the biomass group (21.3% vs 5%, P<.0001), although this difference disappeared when corrected for gender. The emphysema phenotype was more common in the tobacco group (45.9% vs 31.9%, P=.009). The prevalence of chronic bronchitis, exacerbator phenotypes, the comorbidity burden and the rate of hospital admissions were the same in both groups.
ConclusionDifferences were observed between COPD caused by biomass and COPD caused by tobacco smoke, although these may be attributed in part to uneven gender distribution between the groups.
La exposición al humo de biomasa es un factor de riesgo para enfermedad pulmonar obstructiva crónica (EPOC). Se ignora si la EPOC por biomasa y por tabaco tienen características diferentes.
ObjetivoBuscar diferencias clínicas entre ambos tipos de enfermedad.
MétodosEstudio observacional retrospectivo de 499pacientes diagnosticados de EPOC por biomasa o por tabaco. Se compararon ambos grupos respecto a variables clínicas.
ResultadosCiento veintidós sujetos (24,4%) fueron clasificados en el grupo de biomasa y 377 (75,5%) en el de tabaco. El porcentaje de varones fue más alto en el grupo de tabaco (91,2% vs 41,8%, p<0,0001) y la edad resultó inferior en este grupo (70,6 vs 76,2años, p<0,0001). Los valores del índice de masa corporal y del FEV1% fueron superiores en el grupo de biomasa (29,4±5,7 vs 28,0±5,1; p=0,01 y 55,6±15,6 vs 47,1±17,1; p<0,0001, respectivamente). El fenotipo mixto EPOC-asma fue más prevalente en el grupo biomasa (21,3% vs 5%, p<0,0001), aunque esta diferencia desapareció al hacer una corrección por sexo. El fenotipo enfisema fue más frecuente en el grupo tabaco (45,9% vs 31,9%, p=0,009). La prevalencia de los fenotipos bronquitis crónica y exacerbador, el peso de las comorbilidades y la tasa de ingresos hospitalarios fueron equivalentes entre los 2grupos.
ConclusiónExisten diferencias clínicas entre la EPOC por humo de biomasa y por tabaco, aunque podrían ser atribuibles en parte a desigualdades de sexo entre ambos grupos.
Although tobacco smoke is widely recognized as the main risk factor for chronic obstructive pulmonary disease (COPD), a relatively high percentage of COPD patients in international studies are never-smokers.1,2 This proportion is particularly high in developing countries, but it is also significant in Europe. In Spain, the IBERPOC found that 24.3% of COPD patients had never smoked.3 There are several risk factors for the disease that are unrelated to tobacco smoking, with environmental contamination by biomass smoke in enclosed spaces being one of the most important. Indeed, around half of the world's population is exposed to biomass fuel, suggesting that this may be the most significant risk factor for developing COPD worldwide.1 Various epidemiological studies, including one performed in Spain, confirm the association between biomass smoke exposure and COPD.4–6
It remains unclear if COPD due to biomass smoke and that caused by tobacco smoke have different characteristics. It is unknown whether both types of disease have a similar clinical presentation, whether the natural history of the disease is the same, whether patients have similar comorbid conditions and whether pulmonary and systemic inflammatory patterns are similar.7
At present, the term COPD is understood to encompass a series of entities with different characteristics, and the importance of defining clinical phenotypes for the classification of patients into subgroups with varying prognostic and therapeutic implications for the clinical management of patients and the conduct of clinical trials has been emphasized.8,9
The working hypothesis of the authors is that tobacco smoke and biomass smoke may produce different biological effects that would give rise to a distinctive clinical presentation in each subtype. Clinical features that may differ between the 2 types could be grouped into phenotypes and determine the need for different treatments. Comorbidities associated with COPD may also be different in each of the groups, since biomass smoke may not have the same effect as tobacco smoke in the development of disorders such as cardiovascular or malignant diseases. The main objective of this study has been to identify clinical differences between patients with COPD caused by tobacco smoke and by exposure to biomass smoke. Specifically, an attempt has been made to determine differences in the prevalence of the various pre-defined clinical phenotypes and in comorbidities between both groups.
MethodsSubjects and Study DesignThis is a descriptive, retrospective study performed in the Pulmonology Department of a university hospital attending a population of 220000 inhabitants, many of whom live in rural areas in which biomass fuels (mainly wood) are commonly used for cooking and heating. The clinical records of 529 consecutive patients seen in a dedicated clinic in the hospital and diagnosed with COPD between January 2009 and June 2013 were retrospectively reviewed. Subjects were selected from a healthcare database that included patients diagnosed by a pulmonologist as having COPD associated with tobacco use, biomass smoke or alpha-1 antitrypsin deficiency. Inclusion criteria for subjects in this study were age ≥40 years, post-bronchodilator FEV1/FVC ratio <0.70, chronic cough, sputum production or dyspnea, and either a history of tobacco smoking or significant exposure to biomass smoke. Exclusion criteria were alpha-1 antitrypsin deficiency, cystic bronchiectasis or cylindrical bronchiectasis attributed to a cause other than COPD, human immunodeficiency virus infection, concomitant interstitial lung disease, current diagnosis of asthma, history of workplace exposure to inorganic dust or types of smoke other than those produced by burning tobacco or biomass, and parenchymal lung disease associated with previous tuberculosis. The study was approved by the ethics committee of the center (Clinical Research Ethics Committee of Galicia, Registry No. 2012/132).
Accumulated exposure to biomass smoke was difficult to calculate, since it often varied over time. Many patients were exposed during their childhood and youth to smoke in the environment from the traditional kitchens used in the region (open fires in a fireplace). These fireplaces produce more contamination than the ovens and stoves that have generally replaced them in recent decades. Moreover, exposure to smoke is significantly higher in the winter months than the rest of the year and varies from year to year with changing climatic conditions. This, and the retrospective nature of the study, made it impossible to accurately estimate smoke exposure in terms of hours/year. However, a population study carried out in over 5000 subjects showed that cooking for 10 years or more over a wood fire was an independent risk factor for COPD.10 Considering that the study population used biomass not only for cooking but also for heating, and that the latter use may produce less environmental contamination, a conservative attitude was adopted for the purpose of this study and a history of at least 20 years exposure to biomass smoke beginning in childhood was considered significant. Patients were assigned to 2 groups: (1) tobacco group (consumption history of at least 10 pack years), and (2) biomass group (significant exposure to biomass smoke as previously defined, and no tobacco smoking history). For analytical purposes, subjects with a history of smoking were assigned to group 1, even if there was a remote history of exposure to biomass smoke.
Airflow obstruction severity was classified according to the GOLD criteria, on the basis of post-bronchodilator FEV1, from GOLD 1 to GOLD 4.11 Patients were categorized into 4 groups (GOLD A–D), according to the combined COPD assessment classification recommended by the GOLD initiative,11 using the Medical Research Council modified dyspnea scale. The BODEx multidimensional index was calculated for each patient according to their situation on their first visit to the pulmonology clinic. This rating assigns different scores depending on body mass index, degree of airway obstruction, severity of dyspnea and number of severe COPD exacerbations.12 Patient comorbidities were evaluated according to the Charlson index with no adjustment for age13 and a specific COPD comorbidity index (COTE).14
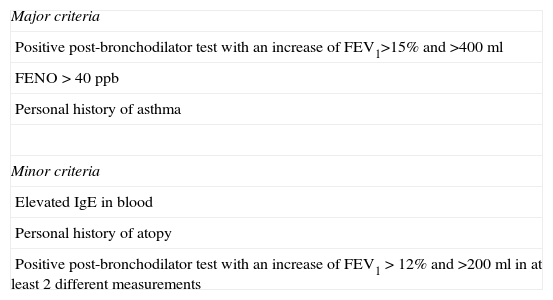
All patients were classified into 3 mutually exclusive phenotypes, using a modified version of the Spanish COPD classification guidelines (GesEPOC)9: (1) chronic bronchitis: cough and sputum production for at least 3 months in 2 consecutive years15; (2) emphysema: no habitual cough and expectoration, (2.1) pulmonary emphysema revealed on computed tomography (CT), or (2.2) reduced CO diffusion (TLCO/VA<80%), or (2.3) chest X-ray suggestive of emphysema16; and (3) mixed COPD-asthma phenotype (MCAP): 2 major criteria or 1 major criterion and 2 minor criteria, as specified in Table 1.
Criteria for the Diagnosis of the Mixed COPD-Asthma Phenotype Used in the Study.
| Major criteria |
| Positive post-bronchodilator test with an increase of FEV1>15% and >400ml |
| FENO>40ppb |
| Personal history of asthma |
| Minor criteria |
| Elevated IgE in blood |
| Personal history of atopy |
| Positive post-bronchodilator test with an increase of FEV1>12% and >200ml in at least 2 different measurements |
FENO: fractional exhaled nitric acid.
Adapted from Soler-Cataluña et al.17
Criteria for the diagnosis of MCAP are based on expert consensus.17 The original criteria included eosinophilia in sputum instead of elevated fractional exhaled nitric acid (FENO), but no facilities for determining eosinophils in sputum are available in the study center, so elevated FENO values were used as a marker suggesting eosinophil inflammation. FENO measurement was performed using a portable device (NIOX MINO, Aerocrine AB, Solna, Sweden) with an oral flow of 50ml/s for 10s. A value greater than 40ppb was considered sufficiently elevated.
Furthermore, all patients were classified according to another clinical phenotype that did not exclude the ones mentioned above: the frequent exacerbator.18 This was defined as presentation of at least 2 exacerbations requiring treatment with antibiotics or corticosteroids (or both) and hospitalization over the year before the first visit to the COPD clinic.
Statistical AnalysisThe data were evaluated for adjustment to a normal distribution using the D’Agostino-Pearson test. For continuous variables, comparison between 2 groups was made using the Student's t-test or Mann–Whitney, as applicable. For comparisons between more than 2 groups, ANOVA or Kruskal–Wallis tests were used. The chi-squared test was used for discrete variables. Data were reported as percentages for discrete variables and mean±standard deviation or median (interquartile range, IQR) for continuous variables, depending on whether they followed a normal distribution or not, respectively. Significance tests were 2-tailed, and a P-value <.05 was considered statistically significant. As this was a retrospective study based on all patients seen in the COPD clinic, the sample size was not calculated beforehand. The statistical power of the study was calculated to detect differences in phenotype prevalence on a 2-tailed basis with an alpha error of 0.05.19
ResultsA total of 529 patients meeting the study inclusion criteria were evaluated. Thirty (30) were excluded due to incomplete data on the study variables. Data on all parameters were retrieved for the remaining 499 patients (94.3%), who were included in the analysis. Of these, 122 (24.4%) were assigned to the biomass group and 377 (75.5%) to the tobacco smoke group.
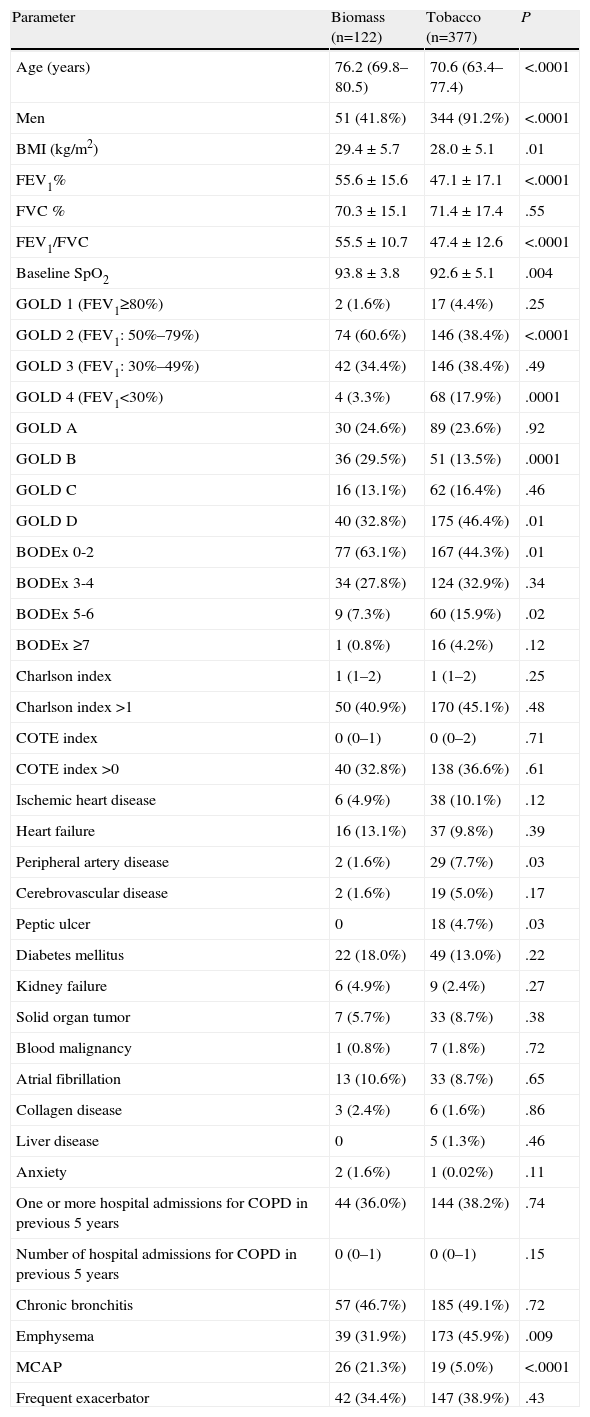
The statistical power of the study to detect a 15% difference (between 30% and 45%) in the prevalence of 2 phenotypes between both groups was 84.5%. The mean age of the study subjects was 72 years (IQR: 64–78) and 395 subjects (79.1%) were men. Patients in the biomass group had been exposed to this smoke for a mean of 62.5±9.9 years. The accumulated tobacco burden for smokers was 60.1±31.4 pack years. The differences between the groups are shown in Table 2. The biomass group was older and included more men than in the tobacco group. Body mass index and baseline pulse oximeter O2 saturation (SpO2) were significantly higher in the biomass group. Biomass patients had less airway obstruction: FEV1% compared to the predicted value was significantly higher, and significantly more patients in this group were classified as GOLD stage 2 than in the tobacco group, while there were fewer GOLD stage 4 subjects in the biomass group. Moreover, using the combined classification of disease severity, there were more GOLD stage B cases in the biomass group, while the percentage of GOLD stage D patients was greater in the tobacco group. In addition, when the patients were classified according to the BODEx index, the biomass group contained significantly more cases with a lower score (0–2), while the percentage of subjects with higher scores (5–6) was greater in the tobacco group. Hospitalization rates for COPD were similar in both groups, and the comorbidity burden, evaluated using the Charlson and COT indexes, was also similar. Peripheral artery disease and peptic ulcer were the most common comorbid conditions in the tobacco group, and this appeared to be associated with an uneven gender distribution in both groups: all cases of peptic ulcer and all cases of peripheral artery disease except 1 occurred in men. No differences were found for other comorbidities (Table 2).
Differences Between Biomass and Tobacco Groups.
| Parameter | Biomass (n=122) | Tobacco (n=377) | P |
| Age (years) | 76.2 (69.8–80.5) | 70.6 (63.4–77.4) | <.0001 |
| Men | 51 (41.8%) | 344 (91.2%) | <.0001 |
| BMI (kg/m2) | 29.4±5.7 | 28.0±5.1 | .01 |
| FEV1% | 55.6±15.6 | 47.1±17.1 | <.0001 |
| FVC % | 70.3±15.1 | 71.4±17.4 | .55 |
| FEV1/FVC | 55.5±10.7 | 47.4±12.6 | <.0001 |
| Baseline SpO2 | 93.8±3.8 | 92.6±5.1 | .004 |
| GOLD 1 (FEV1≥80%) | 2 (1.6%) | 17 (4.4%) | .25 |
| GOLD 2 (FEV1: 50%–79%) | 74 (60.6%) | 146 (38.4%) | <.0001 |
| GOLD 3 (FEV1: 30%–49%) | 42 (34.4%) | 146 (38.4%) | .49 |
| GOLD 4 (FEV1<30%) | 4 (3.3%) | 68 (17.9%) | .0001 |
| GOLD A | 30 (24.6%) | 89 (23.6%) | .92 |
| GOLD B | 36 (29.5%) | 51 (13.5%) | .0001 |
| GOLD C | 16 (13.1%) | 62 (16.4%) | .46 |
| GOLD D | 40 (32.8%) | 175 (46.4%) | .01 |
| BODEx 0-2 | 77 (63.1%) | 167 (44.3%) | .01 |
| BODEx 3-4 | 34 (27.8%) | 124 (32.9%) | .34 |
| BODEx 5-6 | 9 (7.3%) | 60 (15.9%) | .02 |
| BODEx ≥7 | 1 (0.8%) | 16 (4.2%) | .12 |
| Charlson index | 1 (1–2) | 1 (1–2) | .25 |
| Charlson index >1 | 50 (40.9%) | 170 (45.1%) | .48 |
| COTE index | 0 (0–1) | 0 (0–2) | .71 |
| COTE index >0 | 40 (32.8%) | 138 (36.6%) | .61 |
| Ischemic heart disease | 6 (4.9%) | 38 (10.1%) | .12 |
| Heart failure | 16 (13.1%) | 37 (9.8%) | .39 |
| Peripheral artery disease | 2 (1.6%) | 29 (7.7%) | .03 |
| Cerebrovascular disease | 2 (1.6%) | 19 (5.0%) | .17 |
| Peptic ulcer | 0 | 18 (4.7%) | .03 |
| Diabetes mellitus | 22 (18.0%) | 49 (13.0%) | .22 |
| Kidney failure | 6 (4.9%) | 9 (2.4%) | .27 |
| Solid organ tumor | 7 (5.7%) | 33 (8.7%) | .38 |
| Blood malignancy | 1 (0.8%) | 7 (1.8%) | .72 |
| Atrial fibrillation | 13 (10.6%) | 33 (8.7%) | .65 |
| Collagen disease | 3 (2.4%) | 6 (1.6%) | .86 |
| Liver disease | 0 | 5 (1.3%) | .46 |
| Anxiety | 2 (1.6%) | 1 (0.02%) | .11 |
| One or more hospital admissions for COPD in previous 5 years | 44 (36.0%) | 144 (38.2%) | .74 |
| Number of hospital admissions for COPD in previous 5 years | 0 (0–1) | 0 (0–1) | .15 |
| Chronic bronchitis | 57 (46.7%) | 185 (49.1%) | .72 |
| Emphysema | 39 (31.9%) | 173 (45.9%) | .009 |
| MCAP | 26 (21.3%) | 19 (5.0%) | <.0001 |
| Frequent exacerbator | 42 (34.4%) | 147 (38.9%) | .43 |
MCAP: mixed COPD-asthma phenotype; BMI: body mass index.
Results are expressed as number of cases (percentage), mean±standard deviation or median (interquartile range).
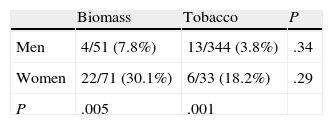
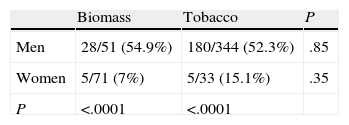
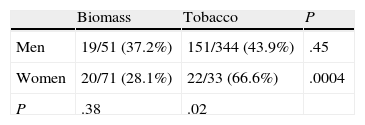
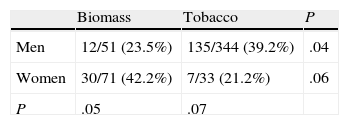
The distribution of chronic bronchitis and frequent exacerbator phenotypes were similar in both groups. MCAP was more common in the biomass group, while the emphysema phenotype was more frequently diagnosed in the tobacco group. An emphysema phenotype was diagnosed from the chest CT in 120 of 212 cases (56.6%) and from reduced CO diffusion in 28 additional cases. Absence of criteria for chronic bronchitis and a compatible chest X-ray were the basis for this diagnosis in the remaining 64 cases. To determine if differences in phenotype prevalence were attributable to sex, patients from the biomass and tobacco groups were classified within each phenotype by gender (Tables 3–6). The differences observed in the distribution of the MCAP appeared to be clearly related to sex; this phenotype was more frequently diagnosed in women, both in the biomass group and in the tobacco group (Table 3). In contrast, when patients were classified by gender, there was no significant difference in the prevalence of this phenotype between both types of exposure. Similar results were found for the chronic bronchitis phenotype; this phenotype was diagnosed more frequently in men than in women, in both the biomass and the tobacco group, while there were no differences in gender in the frequency of diagnosis of this phenotype between both types of smoke exposure (Table 4).
The emphysema phenotype appeared to be more prevalent in the tobacco group because of its increased incidence among the group of female smokers (Table 5). The exacerbator phenotype was more common in male smokers, while it occurred at a similar rate in women exposed to both types of smoke (Table 6). In the overall group of patients exposed to biomass smoke, the exacerbator phenotype was more common among women; this difference tended toward significance.
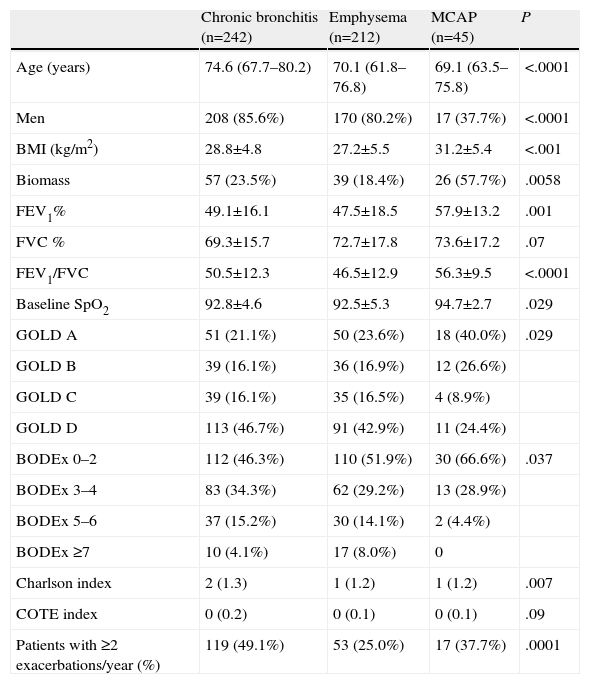
Patient characteristics classified by phenotypes are presented in Table 7. Numerous differences were found in patient characteristics when they were classified in this way.
Differences Between Clinical Phenotypes.
| Chronic bronchitis (n=242) | Emphysema (n=212) | MCAP (n=45) | P | |
| Age (years) | 74.6 (67.7–80.2) | 70.1 (61.8–76.8) | 69.1 (63.5–75.8) | <.0001 |
| Men | 208 (85.6%) | 170 (80.2%) | 17 (37.7%) | <.0001 |
| BMI (kg/m2) | 28.8±4.8 | 27.2±5.5 | 31.2±5.4 | <.001 |
| Biomass | 57 (23.5%) | 39 (18.4%) | 26 (57.7%) | .0058 |
| FEV1% | 49.1±16.1 | 47.5±18.5 | 57.9±13.2 | .001 |
| FVC % | 69.3±15.7 | 72.7±17.8 | 73.6±17.2 | .07 |
| FEV1/FVC | 50.5±12.3 | 46.5±12.9 | 56.3±9.5 | <.0001 |
| Baseline SpO2 | 92.8±4.6 | 92.5±5.3 | 94.7±2.7 | .029 |
| GOLD A | 51 (21.1%) | 50 (23.6%) | 18 (40.0%) | .029 |
| GOLD B | 39 (16.1%) | 36 (16.9%) | 12 (26.6%) | |
| GOLD C | 39 (16.1%) | 35 (16.5%) | 4 (8.9%) | |
| GOLD D | 113 (46.7%) | 91 (42.9%) | 11 (24.4%) | |
| BODEx 0–2 | 112 (46.3%) | 110 (51.9%) | 30 (66.6%) | .037 |
| BODEx 3–4 | 83 (34.3%) | 62 (29.2%) | 13 (28.9%) | |
| BODEx 5–6 | 37 (15.2%) | 30 (14.1%) | 2 (4.4%) | |
| BODEx ≥7 | 10 (4.1%) | 17 (8.0%) | 0 | |
| Charlson index | 2 (1.3) | 1 (1.2) | 1 (1.2) | .007 |
| COTE index | 0 (0.2) | 0 (0.1) | 0 (0.1) | .09 |
| Patients with ≥2 exacerbations/year (%) | 119 (49.1%) | 53 (25.0%) | 17 (37.7%) | .0001 |
BMI: body mass index.
Results are expressed as median (interquartile range), number of cases (percentage), or mean±standard deviation.
Patients with the chronic bronchitis phenotype were older. As mentioned above, there were more women in the MCAP phenotype group. Body mass index and the percentage of subjects exposed to biomass smoke were higher in the MCAP group. FEV1 and baseline SpO2 were higher in the MCAP group. Of the chronic bronchitis and emphysema phenotypes, more patients were GOLD group D, while more of the MCAP phenotypes were GOLD group A. More patients in the MCAP group achieved a low score (0–2) on the BODEx index, and more chronic bronchitis and emphysema phenotypes had higher scores (5–6). The Charlson index was higher in the chronic bronchitis phenotype. There was no significant difference between the COTE index and FVC among the various groups.
DiscussionA great number of scientific articles have been published on COPD, but there are relatively few papers addressing the association between this disease and exposure to biomass smoke. Some earlier studies suggest that patients with COPD caused by biomass smoke may have distinct clinical characteristics, but so far little information on the extent of these differences and the possible therapeutic implications is available. Hypothetically, biomass smoke and tobacco smoke may cause different biological effects, and as a result the disease may have a dissimilar course in aspects such as rate of progression of airway obstruction, degree of airway and parenchymal involvement, types of related comorbidities, etc. The main objective of this study was to determine if there were differences in the prevalence of certain characteristics of COPD due to biomass smoke that could be grouped into phenotypes for easy recognition in daily clinical practice, prompting a different therapeutic approach. Another point of interest was to identify differences in the prevalence of comorbidities, since these have shown prognostic value in this disease.14
The percentage of patients with COPD attributed to biomass smoke in this study (24.4%) is consistent with the proportion of cases of COPD in non-smokers reported in national and international studies.1,3 It must be pointed out that in this study, an attempt was made to exclude airflow obstruction caused by other noxious substances, such as workplace exposure to inorganic dusts or other smokes or vapors. For this reason, the percentage of COPD caused by biomass smoke may have been rather lower, but this has a relative value, since this research project was limited to a small geographical region, and the IBERPOC study revealed significant regional differences in the prevalence of the disease.3
This study revealed some clinical differences between subjects with COPD caused by tobacco smoke and COPD attributable to biomass smoke. It is not surprising that the proportion of women in the biomass group is much higher, since women have traditionally taken on the task of cooking, so their level of exposure to contaminants in biomass smoke is greater. Patients in the biomass group were older, as has also been reported in other studies.20 There are several explanations for this. Although exposure to biomass smoke generally begins at an early age, it is typically intermittent, and the highest levels of contaminant inhalation are only reached during cooking. Thus, damage to the respiratory tract with biomass smoke may possibly develop at a slower rate than with the less sporadic exposure tobacco smoke. It may be that the harmful effects of biomass smoke progress more slowly than tobacco smoke due to biological differences in its composition. The less severe airway obstruction in the biomass group, also found in other studies,20 would support this notion. The diagnosis of COPD due to biomass smoke may also be delayed, since clinicians have less information on this association than they do for tobacco. This hypothesis appears to be less likely; however, it can be possible if it is taken into consideration that the severity of the disease, determined with several markers, was lower in the biomass group. If diagnosis was delayed, patients might be expected to present in a more advanced disease stage. Finally, differences may also be influenced by a bias in survival, although this hypothesis also seems unlikely, since previous studies have shown similar mortality rates in patients with tobacco and biomass COPD, after correction for possible confounding factors.20 Due to the study design, all these hypotheses are merely speculative.
To the authors’ knowledge at the time of writing, this is the first study to examine the distribution of COPD clinical phenotypes associated with biomass smoke exposure. The main finding of this study is a greater prevalence of MCAP in the biomass group. However, this appears to be related to a higher percentage of women in this group, suggesting that the difference is not due to a different biological response to the different types of smoke, but rather to gender-dependent changes in the clinical presentation of the disease. In this regard, a recent article from Izquierdo-Alonso et al.21 should be mentioned, in which the characteristics of 3 clinical phenotypes in tobacco-related COPD were studied: MCAP was also reported to be more common among women.
Another significant finding from this study was a greater prevalence of the emphysema phenotype in the tobacco group, principally due to the greater incidence of this phenotype in the group of female smokers. Interestingly, a pathology study carried out in women with COPD showed that tobacco smokers had more emphysema and caliciform cell metaplasia than those in the biomass group, who had more pigment deposits in the pulmonary parenchyma and more small airway fibrosis.22 Similarly, a study performed in Colombia comparing 12 women with COPD because of wood smoke with 10 women with COPD because of tobacco smoke and similar grades of airway obstruction, found significantly higher emphysema scores (evaluated by high-resolution CT [HRCT]) in the biomass group than in the tobacco group, while the tobacco group more frequently presented peribronchiolar thickening and bronchial dilations.23 A very recent study in 43 women with COPD (21 from biomass smoke and 22 from tobacco) matched for age and FEV1 also found higher emphysema scores on HRCT in the tobacco group and greater levels of air trapping in the biomass group.24 Taken together, these results may reveal a greater risk of emphysema from exposure to tobacco smoke than to biomass smoke, a risk that may also be heightened in women.
The distribution of the chronic bronchitis phenotype was the same in the biomass and tobacco groups, although it was more common in men than in women. In contrast, a Chinese study found that patients with COPD who had never smoked had less cough and sputum production than smokers.25 However, this article did not directly address COPD from biomass smoke. In contrast, Ramírez-Venegas et al.20 did not find any differences in the percentages of patients with cough and moderate/severe expectoration between the biomass and tobacco COPD groups.
In this study, no significant differences were found between the 2 study groups for the frequent exacerbator phenotype. Few studies have so far evaluated this issue. In their study, Ramírez-Venegas et al.20 found no differences between the number of exacerbations in patients with COPD caused by tobacco or by biomass smoke, and the incidence of patients seen in emergency rooms and hospital admissions were similar in both groups. Similarly, in this study no significant differences were found in the number of hospital admissions due to COPD exacerbations.
Another of the question that this study aimed to address was the prevalence of different comorbidities in the 2 study groups. This issue is important due to its connotations for prognosis.14 Additionally, significant differences between groups in the rates of different comorbidities may provide evidence of a different biological effect for both types of smoke. No variations in comorbidities in these 2 groups were observed that could support this hypothesis. Comorbidity burden, calculated with 2 indexes validated for prognostic significance, was similar between the 2 groups and the various comorbid conditions were distributed similarly. Only peripheral artery disease and peptic ulcer occurred more frequently in the tobacco group, but once again, this appears to be associated with differences in gender, since these disorders were observed practically exclusively in men.
Although it was not the main aim of the study, it is worth mentioning the differences between the various defined phenotypes, paying particular attention to the lower severity, in general, of MCAP and the lower percentage of exacerbator patients in the emphysema phenotype.
Definitions of the phenotypes used in this study should be discussed in some detail. It is now recognized that FEV1 alone does not provide an adequate description of the heterogeneity of COPD. Proposals have been made to define subgroups of patients with similar disease characteristics related with significant outcomes, for clinical and investigational purposes.8 The GesEPOC guidelines recommend classifying patients into several predefined phenotypes that may have prognostic and therapeutic implications, but these phenotypes have not yet been fully validated.26 In this study, a classic definition of chronic bronchitis was used that has been shown in several studies to be predictive of a poor prognosis, including a greater risk of exacerbations.27,28 As in other studies,18 the definition of the frequent exacerbator phenotype was based on the history of previous exacerbations, and has been observed to be relatively stable over time.18 The identification of this phenotype is especially relevant, since recurrent exacerbations are associated with a poor quality of life, accelerated loss of pulmonary function, and higher death rates.29,30
The definitions used for the emphysema and MCAP phenotypes are more controversial. Chest CT was performed in slightly over half of the patients, and many of the emphysema phenotype cases were identified on the basis of a reduced TLCO/VA and/or compatible chest X-ray, in the absence of clinical criteria for chronic bronchitis. Although Miniati et al.16 showed that chest X-ray is acceptably sensitive and specific for the detection of emphysema, and that it is useful for phenotyping COPD, the emphysema phenotype may have been overdiagnosed in this study. The definition of MCAP is based on a consensus document that has not yet been validated.17 Moreover, a variation of the definition proposed for this phenotype has been used, as elevated FENO values were used instead of eosinophilia in sputum. Finally, the phenotypes defined, with the exception of frequent exacerbator, were mutually exclusive. Notwithstanding, Marsh et al.31 found that 37.5% of COPD patients had a combination of more than 1 phenotype.
This study has several limitations. The most obvious are the retrospective design, conduct in a single study center, and a relatively small sample size. Regarding the latter, the study had sufficient power to detect differences in the prevalence of the different phenotypes of the magnitude observed between MCAP and emphysema, but it may have lacked power for detecting smaller differences. The retrospective character of the study is the main drawback, and some error in the classification of the clinical phenotypes is possible. Nevertheless, all patients were managed by the same team, who had adopted the practice of systematically classifying patients by phenotypes when they are seen in the clinic, so significant errors in this respect are unlikely. The possibility of some overlap between the tobacco and biomass groups is more relevant, since smokers with a remote history of exposure to biomass smoke were assigned to the tobacco group. In addition, patients were recruited in a hospital Pulmonology Department, so a bias in selection is inevitable. Indeed, there were more patients in GOLD group D than in groups A to C, and the prevalence of the frequent exacerbator phenotype was relatively high. As such, the study population cannot be considered representative of the whole disease spectrum. Moreover, as mentioned in the methods section, the level of exposure to biomass smoke was difficult to estimate, more so because of the retrospective character of the study. In consequence, a dose–response relationship could not be established between exposure to smoke and the severity of the disease and/or comorbidities. As discussed above, the definition of MCAP used in this paper may be controversial, and the prevalence of this phenotype could vary significantly if another definition was used. The lack of general consensus on the definition of this phenotype makes it difficult to compare this study with those published by other authors. Although workplace exposure to mineral dusts and other toxic smoke was excluded from this study, many of the patients exposed to biomass smoke were farm workers and livestock breeders who, as such, were exposed to organic dusts and gases that have been associated with the development of chronic airway limitation.32 This confounding factor, also observed in previous studies on biomass COPD performed primarily in rural environments, is very difficult to eliminate. Socioeconomic factors that may be associated with poorer pulmonary function comprise another possible confounding factor that is difficult to avoid in this population.
Despite its limitations, this study identifies some differences between biomass and tobacco COPD that may demonstrate different biological effects caused by the 2 types of smoke. Biomass smoke may be less harmful and cause less severe airflow limitation with later onset. It has already been suggested in previous studies that tobacco smoke may cause a higher grade of emphysema and this should be confirmed in larger, prospective studies, also designed to clarify the possibly complex associations between the effect of one or other type of smoke and the difference in gender response to inhalation of different harmful substances.
Conflict of InterestThe authors state that they have no conflict of interest with respect to this article.
Please cite this article as: Golpe R, Sanjuán López P, Cano Jiménez E, Castro Añón O, Pérez de Llano LA. Distribución de fenotipos clínicos en pacientes con enfermedad pulmonar obstructiva crónica por humo de biomasa y por tabaco. Arch Bronconeumol. 2014;50:318–324.