This new update of the recommendations of the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) was written for the purpose of broadening the knowledge on community-acquired pneumonia (CAP) in its epidemiological, diagnostic, therapeutic and preventive aspects by presenting new systematized information based on current scientific evidence. In order to elaborate these recommendations, the authors distinguished 6 sections: epidemiology, assessment of the severity and prognostic scales, microbiological diagnosis, antimicrobial treatment, CAP that does not respond to treatment and prevention. As in previous documents, the recommendations concern cases of CAP that occur in immunocompetent persons and adults, i.e. 18 years of age or older. The infections that affect different sub-populations of subjects with other characteristics (For example: children, patients with cancer or other immunosuppressive conditions, institutionalized patients, etc.) require a different assessment which is not covered under this guideline.
At the end of the document and before the bibliography, a summary of the recommendations is presented with the corresponding quality of evidence for each one. In short, Level 1 evidence consists of the following types of studies: meta-analysis and systematic reviews of randomised clinical trials (RCT) or RCT with different risks of bias; Level 2: high-quality systematic reviews of cohort studies or cases and controls, or cohort studies or cases and controls that are well-conducted with different levels of the risk of confusion; Level 3: non-analytical studies (clinical observations and case series) and Level 4: expert opinions. Furthermore, the strength of the recommendation was also taken into consideration (high, moderate, low, very low) in the explicit statement of key points.1
EpidemiologyIncidenceThe prospective population studies estimate the annual incidence of CAP to be between 5 and 11% of the adult population.2,3 It is well-known that the disease is more common in males, in the extremes of life, in winter and in the presence of various risk factors, including tobacco and alcohol consumption, malnutrition, uraemia or chronic obstructive pulmonary disease (COPD).4 The number of hospitalisations varies between 1.1 and 4 per 1,000 patients in different countries. Among other causes, this variability could be according to the differences in patient care in the primary health care setting or the specialized health care setting.5 Furthermore, the number of hospitalisations increase with age (1.29 per 1,000 in patients from 18 to 39 years of age versus 13.21 per 1,000 in those who are 55 years old or older).5 Meanwhile, between 1.2 and 10% of hospitalised patients due to CAP require hospitalisation in an intensive medical unit.
MortalityMortality can vary from 1 to 5% in ambulatory patients, 5.7 to 14% in hospitalised patients and from 34 to 50% in those hospitalised in an intensive care unit (ICU)6 especially in patients who need assisted ventilation.7 Mortality in the intermediate and long term is high with figures showing 8% at 90 days, 21% per year and 36% at the end of 5 years.8
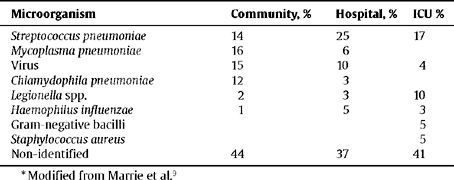
AetiologyThe most common aetiological findings in ambulatory and hospitalised patients are shown in Table 1 although the pathogenic agent is unknown in a large number of cases. The most common cause in all the series and in all the areas is Streptococcus pneumoniae.9 The incidence of Mycoplasma pneumoniae can depend upon whether or not the study was conducted in epidemic years. Staphylococcus aureus, Legionella spp. and resistant pneumococci are common in hospitalised ICU patients.10 Gram-negative enteric bacilli (GNEB), Chlamydophila psittaci and Coxiella burnetii are rarely the cause of CAP. The incidence of polymicrobial infections vary from 5.7 to 13%, depending upon the type of study and the intensity of the search for the causal agents11 (Table 1).
Distribution of the potential aetiologies of CAP *
| Microorganism | Community, % | Hospital, % | ICU % |
| Streptococcus pneumoniae | 14 | 25 | 17 |
| Mycoplasma pneumoniae | 16 | 6 | |
| Virus | 15 | 10 | 4 |
| Chlamydophila pneumoniae | 12 | 3 | |
| Legionella spp. | 2 | 3 | 10 |
| Haemophilus influenzae | 1 | 5 | 3 |
| Gram-negative bacilli | 5 | ||
| Staphylococcus aureus | 5 | ||
| Non-identified | 44 | 37 | 41 |
Even though in different studies the definitions of elderly or advanced aged patients are not homogenous, in general, M. pneumoniae, Legionella spp. and GNEB are less common in the geriatric population. In contrast, Haemophilus influenzae and episodes of aspiration pneumonia have been described more frequently in this population.12
Aetiology in Patients With COPDInfections due to H. influenzae and Moxarella catarrhalis as well as bacteraemias caused by pneumococci are more common in patients with COPD. In studies conducted in Spain, an aetiological distribution similar to that of the general population has been described such as an increase in infections caused by S. pneumoniae, enterobacteria, Pseudomonas aeruginosa and mixed infections.13
Aetiology in Persons Living in Nursing Homes for Senior CitizensIn a CAP study conducted in Spain, specifically on healthcare-associated pneumonia (HCAP) that included 25.4% of patients living in nursing homes, a greater incidence of aspiration pneumonia was described due to H. influenzae, GNEB and S. aureus. Legionella spp. and cases of unknown aetiology were less common.14 However, in a prospective study of cohorts conducted in the United Kingdom which compared acquired pneumonia in nursing homes versus patients over 65 years of age did not show any aetiological differences.15
Clinical ManifestationsThe symptomatology of CAP is unspecific and its diagnosis is based on a group of signs and symptoms related to a lower respiratory tract infection and a compromised general state of health, including fever (>38 °C), cough, expectoration, chest pain, dyspnoea or tachypnoea, and signs of invasion of the alveolar space. Absence of fever, the onset of confusion and worsening of underlying diseases is common in elderly patients.
In general, there is no characteristic, clinical sign or a combination of both that allows a specific aetiology to be deduced or that permits the differentiation of CAP from other lower respiratory tract infections with sufficient reliability. However, infection due to S. pneumoniae is more common in patients of an advanced aged with underlying diseases or with a sudden onset of high fever and chest pain of a pleuritic nature. Similarly, bacteraemias due to pneumococcal CAP occur more commonly in female patients, alcohol consumers, patients with diabetes mellitus, COPD and those who present a nonproductive cough. CAP due to L. pneumophila is more common in younger patients who smoke without any associated co-morbidities and who present symptoms of diarrhoea, signs of serious infection and multisystem neurological involvement. Hyponatremia, hypophosphatemia and haematuria are also related to this microorganism. Meanwhile, CAP due to M. pneumoniae is more common in young patients with multisystem involvement being less common and is more common in patients who were treated with antibiotics prior to the diagnosis of CAP. Viral pneumonias are described with increasing frequency in patients with congestive heart failure.
Laboratory TestsOxygen saturation tests, CBC and basic biochemistry, including renal function tests, liver function tests and electrolytes are recommended in patients with CAP because it provides valuable information about the patient's status and contributes to its classification in different prognostic scales.
Chest X-RayThe presence of infiltration on the chest x-ray in a patient with clinical manifestations suggesting CAP is the gold standard for the diagnosis of this disease. Since the clinical manifestations of CAP are non specific, the chest x-ray is mandatory in order to establish its diagnosis, location, extension, potential complications (pleural effusion or cavitation), the presence of associated pulmonary diseases, other potential alternative diagnoses and also to confirm its evolution towards progression or healing. Bilateral involvement or of two or more lobes and the presence of pleural effusion are indicators of the severity of the disease, especially bilateral pleural effusion either by the pneumonia itself or by associated cardiac insufficiency.
Furthermore, there are no characteristic radiological signs that allow the causal microorganism to be established. A chest CT usually does not provide new information, but can be useful in doubtful cases or as a support tool in the treatment of pleural complications.
Clinical healing precedes the radiological resolution and is slower in elderly patients. Radiological worsening is the most common event that can be observed after hospitalization in patients with Legionella spp. infection, bacteremic pneumococcal pneumonia and in elderly patients.
Initial Assessment of the Severity and Prognostic ScalesThe initial assessment of the severity of the patient with CAP is the crucial key to establishing the treatment and the most appropriate location for care. When choosing hospitalisation as the most appropriate option, the deciding factor as to whether the patient should be treated in a hospital ward, ICU or intermediate respiratory care unit (IRCU) would be early identification of the most severe patients and those whose health status could quickly deteriorate. Obviously, the best results are obtained when the patient benefits from the level of care that is appropriate for each location for their specific individual condition. A delay in determining the severity and, consequently, sub-optimal treatment and care from the time the patient arrives at the hospital and his transfer to ICU is associated with an increase in mortality.16
The use of clinical judgement to assess the severity of CAP depends upon the experience of the attending physician and can underestimate or overestimate the severity of the process. Prognostic scales for severity have been developed to solve this problem whose purpose is to classify patients into difference risk groups according to the probability of death within 30 days or to specify a more aggressive treatment such as assisted ventilation or the administration of vasopressor drugs. The most well-known and useful prognostic scales are the Pneumonia Severity Index (PSI)17 and the CURB65,18 an acronym for Confusion, Urea (urea >7 mml/l), Respiratory rate (RR ≥30), Blood pressure (diastolic BP ≤60 mm Hg or systolic BP <90 mm Hg) and age ≥65 years.
Twenty weighted variables are used to calculate the PSI which includes age, sex, co-morbidities, vital signs and analytical and radiological changes. According to the total score, the patients are stratified into 5 classes (I-V) or categories according to the risk of mortality within 30 days. Classes I-III refer to patients with mild CAP (low risk of death, between 0.1–2.8%), Class IV are patients with an intermediate risk (risk of death between 8.2–9.3%) and Class V are patients at high-risk (risk of death between 27–31%). Treatment is recommended on an outpatient basis for Classes I-II except in cases of hypoxemia (PaO2 <60 mm Hg or 02 Sat <90%), observation in short-stay units for Class III and hospitalization for Classes IV-V.
The British Thoracic Society initially developed the CURB19 and Lim et al18 subsequently redesigned it by incorporating the age and slightly modifying the initial acronym by substituting it with CURB65. The calculation of the final score is carried out by adding one point for each variable present with a range between 0 and 5 points. This scale stratifies patients into three groups or risk classes: 0 to 1 low risk (mortality 1.5%), 2 is an intermediate risk (mortality 9.2%) and 3 to 5 is high-risk (mortality 22%). Hospitalisation is recommended when the score is >1, especially if other factors are present associated with severity such as hypoxemia or multilobar involvement in the chest x-ray.
Once having decided upon hospitalisation, it is helpful to distinguish the patients requiring treatment in ICU or IRCU from those in which conventional hospitalization is sufficient. It is difficult to establish standardised criteria for admission into the ICU and, indeed, there is a great variation in the percentage of patients hospitalised in the intensive care units between the different hospitals (4 and 17%).20 This variability is partly due to the decision that is made by the attending physician using his clinical judgement to admit a patient to ICU and is closely related to local practices.
In an effort to better predict which patients should be treated in the ICU, the American Thoracic Society and the Infectious Diseases Society of America (ATS/IDSA)21 have designed a new severity scale which includes 2 major criteria (invasive mechanical ventilation and septic shock with the need for vasopressor drugs) and 8 minor criteria (RR >30; PaO2/FiO2 <250 mm Hg; multilobar infiltration in the chest x-ray; confusion/disorientation; uraemia >20 mg/dl; leucopoenia [<4,000 leucocytes/mm3]; thrombopoenia [<100,000 platelets/mm3]; hypothermia [<36 °C] and hypotension requiring aggressive fluid therapy). The presence of one major criterion or at least three minor criteria will indicate the need for admission into ICU or in high-level monitoring units. Although the predictive capacity of this instrument to identify severe pneumonias and admission into ICU has been validated,22 the obviousness of the major criteria limits its effectiveness.
In a further attempt to avoid variability in the admission criteria for ICU for patients with CAP, Charles et al23 has recently designed a severity scale focused only to determine the need for respiratory or intensive vasopressor support. This scale, called SMART-COP for the initials of the variables involved in the assessment, consists of 8 clinical and analytical variables with different cut-off points based on age. Each one of the 8 variables is given a score (low systolic blood pressure, 2 points; multilobar involvement, 1 point; low albumin level, 1 point; high respiratory rate, 2 points; tachycardia, 1 point; confusion, 1 point; poor oxygenation, 2 points; low arterial pH, 2 points). According to SMART-COP, the patients are stratified into 4 risk groups based on the need for intensive support: from 0 to 2 points, low risk; 3 to 4 points, moderate risk; 5–6 points, high-risk; over 6 points, very high risk.
The severity scale called Severity Community Acquired Pneumonia (SCAP)24 has also been proposed to predict mortality during hospitalisation and/or the need for mechanical ventilation and/or the onset of septic shock. This scale uses 8 weighted variables: arterial pH <7.3; systolic blood pressure <90 mm Hg; confusion or altered mental status; respiratory rate >30 mg/dl; urea >30 mg/dl; PaO2 <54 mm Hg or PaO2/FiO2 <250 mm Hg; age ≥80 years and multilobar chest radiography involvement. These variables are then grouped together into 2 major variables and 6 minor variables. According to the total score, the patients can be stratified into the following 5 groups or risk classes: low risk, Classes 0–1 (0 to 9 points); intermediate risk, Class 2 (10 to 19 points); high risk, Classes 3–4 (>20 points).
The two latter models (SMART-COP and SCAP) that predict ICU admission or the development of severe adverse events (mechanical ventilation, shock and/or death) which might warrant treatment in ICU need to be validated in different cohort groups and in different geographical areas.
Strategies to Assess the Severity of CAPThe strategy to be used to assess the severity of a patient with CAP has to be able to answer two basic aspects: a) decision-making as to the need for hospitalization, and b) if hospitalization is required, assigning the appropriate service. It is vital to understand that any severity scale represents an additional support tool to clinical judgement which is what ultimately enables the inflexibility of the prediction benchmarks to be individualized and put into their proper perspective by weighing the effect of additional circumstances (i.e. the degree of stability of potential diseases) and to also take into consideration, personal aspects and the social conditioning of each patient. In the most severe cases, the clinical judgement of the physician must rationalise the use of an additional prognostic scale to identify the profile of those patients likely to be placed in critical care units.
Decision to Admit to the HospitalAfter having established the diagnosis of CAP, the first decision to be made is whether or not the patient requires hospitalisation. Approximately 75% of patients with CAP are initially assessed and treated in the Emergency Department of the hospitals where the workload is usually intense. Therefore, when choosing a severity scale, both its potential predictive power and its effectiveness need to be assessed, and specifically, that it results in easy memorization and simple application.
The PSI and CUR65 are more robust, validated and recommended severity scales, having demonstrated that they possess a similar capacity to select patients in terms of the risk of death within 30 days.25,26 Evidently, both scales have limitations in their predictive capacity and the system that they use to establish patients into risk groups is not perfect. At times, the PSI may underestimate the severity of the disease, especially in younger patients without concomitant diseases, probably due to the weight assigned to the age and co-morbidity. The CURB65, in turn, has the drawback of not being validated in patients over 65 years which restricts its use in this population. The arterial oxygen saturation is not assessed either which is an important vital sign that by itself can lead to the establishment of supplemental oxygen therapy as well as the need for hospitalisation. The real difference between both scales comes from the difficulty in its utilisation in daily clinical practice. The PSI uses 20 variables with different weights and is nearly impossible to memorise. Its practical application requires the use of more or less sophisticated computer tools so that after entering the relevant variables using the guided system, the risk class is automatically assigned. The advantage in using the CURB65 is obvious because it is a very simple scale which is easy to memorise and use.
The ease of implementing the severity scales is fundamental on a primary health care level. In this respect, in addition to the ease of use, it is also specified that analytical variables are not incorporated given its potential lack of availability in this medium. Therefore, the most ideal severity scale is the CRB65,18 a reduced variant of the CURB65, in which the urea variable has been eliminated and it has demonstrated an excellent capacity to group the patients together in risk groups based on mortality.27 Each variable is weighted with 1 point in such a way that patients with ≥1 have to be sent to the hospital to complete their assessment. If a chest x-ray and pulse oximetry are available, the presence of multilobar and/or bilateral radiographic involvement or a SpO2 < 92% would also be criteria to send the patient to the hospital.
Placing the Patient with CAP in the Proper Hospital ServiceAlthough both the PSI and the CURB65 are useful in assessing the risk of death, neither one was designed to assess the need for ICU admission. However, the SMART-COP,23 ATS/IDSA,21 or SCAP24 systems are appropriate in early identification of eligible patients requiring inotropic and/or respiratory support and/or admission to ICU. Furthermore, it should be noted that the interpretation of these models must be done with caution because of the lack of adequate external validation studies and because its utilisation has not demonstrated that it improves the outcomes either. Furthermore, its utilisation can be time-consuming, especially if you take into consideration the work load of the hospital emergency departments where they must often use these tools.
In clinical practice, one would proceed as follows: Once the patient is diagnosed with CAP the PSI17 must be swiftly implemented (a software support tool is indispensable) or the CURB6518 in order to make the decision to hospitalise. The attending physician will use the information which facilitates the prognostic scales to complement his clinical judgement. If hospitalisation is appropriate and the severity of the case warrants it, the physician may be inclined to perform a more specific assessment using a second scale (a software support tool is highly recommended) which enables the patient to be quickly placed in the appropriate service based on the care required. The lack of experience of the physicians undergoing training who treat patients with CAP could be compensated, in part, by using software tools which facilitate the consecutive or simultaneous classification with two prognostic scales and would alert them when a specific score is exceeded.
Biological Markers for the Severity of CAPThe abovementioned scales do not take into account the mechanisms of the inflammatory response. Therefore, the role of biomarkers in the inflammatory response and their correlation to the severity of the infection continues to be a subject of growing interest. The most studied biomarkers linked to mortality due to CAP are C-reactive protein and procalcitonin,28 although other biomarkers are also being investigated such as pro-adrenomedullin, neopterin, copeptin and atrial natriuretic pro-peptide (proANP). Its isolated use does not provide advantages over the standard prognostic scales, but the joint use of prognostic scales and biomarkers is seen as a useful tool.29 Regardless of the need for more validation studies that confirm the role of biomarkers in the prognostic scales, the cost of the determination needs to be taken into consideration because at this time, it could be expensive and the results are not always immediate.
Microbiological DiagnosisEarly, rapid and reliable microbiological diagnosis is essential in establishing an appropriate initial antibiotic treatment which is vital in decreasing the high mortality rate from CAP. Nevertheless, even though proper diagnostic techniques are used, the ability to establish the correct aetiological diagnosis is achieved in only 50% of the cases.21 Meanwhile, in a variable number of cases, the aetiology can be mixed30 and there is no epidemiological, clinical or radiographical standard either which is specific enough for certain aetiologies. The causal diagnosis is required in severe cases or when the agent may involve a change in treatment which could be avoided in mild cases. So, when epidemiological and risk factors that suggest uncommon aetiologies are present, the dimensions of the microbiological study to be conducted should be in accordance with the degree of severity of the patient's health status. Furthermore, in cases of delayed resolution or a lack of treatment response, the reassessment should be more complex in the absence of prior microbiological studies. Although S. pneumoniae is the most common causal agent, the geographical variation in the percentage of resistance and the potential allergic reaction to beta-lactams makes it advisable to isolate this agent in a culture to study the sensitivity. From an epidemiological standpoint, it also requires the determination of the most common serotypes in each area in order to design the vaccinations.
Microbiological diagnosis of lower respiratory tract infections presents significant restraints due to its low cost-effectiveness and the difficulty in obtaining proper quality simples. Interpretation of the findings in poor samples can lead to improper diagnosis and treatment errors. In the case of pathogens which could form a part of the commensal flora such as S. pneumoniae, the aetiological diagnosis of certainty will require its isolation in uncontaminated samples such as blood, pleural fluid or lung tissue, or antigen detection in urine. When isolation and/or antigen detection is carried out on respiratory samples obtained by non-invasive techniques, an aetiological diagnosis of probability is established. The new bacterial antigen detection techniques or amplification of nucleic acids allow the causal agent to be detected more quickly and with a higher degree of sensitivity, especially for those pathogens which are difficult to culture. Isolating primary pathogens such as L. pneumophila or Mycobacterium tuberculosis is of value even in poor quality samples.
Blood CulturePerforming blood cultures is required in the diagnosis of severe pneumonia and to make a certainty diagnosis of bacteremic pneumococcal pneumonia or H. influenzae pneumonia as well as carrying out an in vitro culture and sensitivity study. The blood culture must be performed through aseptic venopuncture with two different blood draws on aerobic and anaerobic mediums since pneumococcal lysis is not uncommon, allowing easier isolation of anaerobic microorganisms, in this case, under anaerobic conditions in aspiration pneumonia. Blood cultures are positive in less than 20% of the cases31 and its usefulness is limited in immunocompetent patients.32 Blood cultures are especially important for patients with chronic diseases or those who are infected by the human immunodeficiency virus (HIV) since the incidence of bacteraemia is higher in these subgroups. CAP is the cause in the majority of pneumococcal bacteraemias in adults. Patients with severe pneumonia also have a higher potential for infection and in addition to S. pneumoniae, other infectious pathogens could be S. aureus or gram-negative bacilli. New blood cultures should also be carried out in cases of treatment failure or in the progression of pneumonia especially in patients with risk factors.
Pleural FluidWhen pleural effusion is present, a thoracentesis and aerobic and anaerobic cultures of the pleural fluid obtained are recommended since the occurrence of empyema is one of the main factors associated with poor outcome in CAP.33S. pneumoniae is the most common isolated microorganism, especially when the infection is caused by specific serotypes such as serotype l34 followed by H. influenzae and pyogenic bacteria such as Streptococcus pyogenes or S. aureus. In pleural fluid samples, antigen detection is also indicated (i.e. if the patient has already been treated with antibiotics) or even nucleic acid detection,35 in both cases with a sensitivity of 80% and a specificity higher than 90%.36 One must remember the potential for tuberculous pleuritis which could be confused with parapneumonic effusion.
SputumSputum is the most common respiratory sample obtained although it is also the most problematic in its interpretation since it presents low sensitivity due to the loss of bacteria caused by the delay in processing as well as the presence of aetiological agents which are difficult to culture. Above all, there is the problem of contamination with the oropharyngeal microbes. Furthermore, one must be cautious in the interpretation of the results after antibiotic treatment was initiated. Microscopic screening of the quality of the sample is required for cellular criteria for the purpose of selecting good quality sputum which shows less than 10 epithelial cells per 100x field and more than 25 leukocytes/100x field. When the quality of the sputum is appropriate and the process is rapid, the visualization of a predominant bacterial morphology in the Gram stain (i.e. gram-positive diplococci) suggests probable pneumococcal pneumonia. The isolation of sputum in cultures is considered a probability diagnosis since the colonization of the oropharynx by pneumococci is very common in children under 2 years old and in patients with chronic pulmonary diseases. Other microorganisms that can be predominantly observed in the Gram stain and isolated in cultures are H. influenzae and M. catarrhalis; in this case, the diagnosis is also presumed because these microorganisms can colonise the respiratory tracts especially in patients with chronic diseases. However, when these microorganisms are predominant in the clinical sample, it is indicated in the antibiogram in order to adjust the treatment.
Furthermore, isolating primary pathogens such as L. pneumophila or Mycobacterium tuberculosis is of value even in poor quality samples. This isolation of Legionella requires specific mediums and has little sensitivity involving a slow progression but continues to be indicated whenever possible since antigen detection in urine allow L. pneumophila to be identified with greater sensitivity in serogroup 1. It is also of interest to identify the environmental sources of infection. Culture and isolation of M. pneumoniae and Chlamydophila shows little sensitivity, is difficult and involves a slow process. Therefore alternative techniques are recommended. Pneumonias caused by enterobacteria (i.e. Klebsiella pneumoniae, Escherichia coli), Pseudomonas and other non-fermentative gram-negative bacilli are more frequently of nosocomial origin but they could be the cause of CAP in certain patient groups such as those who are immunosuppressive and those with chronic diseases (diabetes, COPD), as well as cases of prior hospitalisation and its predominant isolation in good quality sputum can have clinical value.
Special attention should be given to the increase in severe necrotising pneumonia caused by methicillin-resistant S. aureus that occurred, above all, in the Americas and is less common in Europe.37 On the other hand, in 10–15% of cases, CAP is secondary to aspiration pneumonia in patients with risk factors (altered consciousness, difficulty in swallowing and mouth sepsis). In this case, a culture is not required because the causal agents and their sensitivity are predictive. The search for Nocardia spp. through selective mediums and prolonged incubation is indicated in patients with underlying disease and/or immunosuppression treatment that develops into pneumonia with a tendency to form necrotising abscesses and cavitation. In this case, a gram stain may be diagnostic.
Samples Obtained Through Bronchoscopic TechniquesObtaining representative samples of the lower respiratory tract, corresponding to the airway or lung segment which is radiologically affected without contamination with the oropharyngeal flora is especially indicated in the diagnosis of nosocomial pneumonia and in the immunosuppressed patient. It is indicated in severe cases of CAP or in treatment failure. The most appropriate sample varies according to the suspected diagnosis and the location of the lesion. The culture is obtained quantitatively (a series of dilutions or seeding with a calibrated loop) because the concentration of bacteria is at least 105 colony-forming units (cfu)/ml in the lower airways, whereas the colonised bacteria is present in lesser quantities. In cases of bronchial brushing, provided that between 0.01 and 0.001 ml of secretions are collected, the isolation of more than 103 cfu/ml in the deposited sample in 1 ml of normal saline represents this quantity. In bronchoalveolar lavage, growth of more than 104 cfu/ml is considered significant since it is based on alveolar secretions diluted in 10 to 100 ml of normal saline. The cut-off points should be interpreted with caution especially if the patient is already receiving antibiotic treatment with one exception which is the isolation of primary pathogens. Isolated microorganisms that can form a part of the commensal flora or colonisation are considered a presumptive diagnosis even though there is strong evidence in the presence of pneumonia. Bronchoalveolar lavage is also the technique of choice for the investigation of Pneumocystis jirovecii and cytomegalovirus in immunosuppressed patients. In selected cases, transbronchial biopsy by means of bronchoscopy, avoiding more invasive techniques such as transthoracic fine needle aspiration which is reserved for the most serious symptoms in which a diagnosis was not possible.
UrineIn cases of infection caused by S. pneumoniae and L. pneumophila, antigenuria tests detect renal excretion of microbial antigens. In terms of the pneumococcal antigen, counterimmunoelectrophoresis (CIE) can be used to detect capsular polysaccharide and immunochromatography which identifies C polysaccharide with a sensitivity of 80%.38 In patients with bronchial colonisation as occurs in COPD and in children under 2 years of age, C polysaccharide can be detected in urine without pneumococci being the causal agent of the respiratory infection, therefore, CIE is recommended for these patients.39 Furthermore, the persistence of urinary antigen excretion associated with prior episodes of pneumonia or exacerbation of COPD should be taken into consideration.40Legionella antigenuria has become the diagnostic method of reference with heat treatment of the urine and concentration being fundamental in order to increase its sensitivity up to values of 80% with a specificity of 100%.41 It is recommended when the epidemiological context suggests this diagnosis in severe pneumonias and in those which do not respond to initial treatment with beta-lactam antibiotics.
SerologySerology is indicated for the diagnosis of M. pneumoniae and Chlamydophila pneumoniae pneumonia (high titres of IgM antibodies in the serum during the acute phase and/or seroconversion in the IgG antibody titre in the serum during the convalescent phase) especially in younger patients. The main drawbacks are that adults may not present an increase in IgM after repeated re-infections and there is a high prevalence of antibodies to C. pneumoniae in the general population. When infection caused by Coxiella burnetti (Q fever) or Francisella tularensis (tularemia) is suspected considering the epidemiological context and in cases where a diagnosis of L. pneumophila could not be established by other techniques, serology is the technique of choice.
Molecular Biology TechniquesMolecular biology techniques are indicated in severe pneumonias in which an aetiological diagnosis could not be established using standard techniques and in centres with the necessary infrastructure and technical experience. The detection of pneumococcal DNA using the polymerase chain reaction (PCR) technique is useful in pleural fluid samples, whereas the sensitivity is low in blood samples.42 Commercialized PCR techniques in real-time for the detection of M. pneumoniae and C. pneumoniae in samples of nasopharyngeal aspirate has significant diagnostic superiority versus a culture or serology. In certain times of epidemics, the detection of respiratory viruses, such as the flu virus, is indicated and samples preferably taken by nasopharyngeal aspiration. In this instance, rapid techniques such as immunofluorescence and immunochromatograpy may have a higher specificity (90–95%), which allow early treatment to be initiated even though the sensitivity is variable (20–65%) according to the quality of the sample and the viral load.43 Sensitivity improves substantially with an adequate selection of patients and during periods of highest prevalence.44 Molecular biology techniques have greater sensitivity and it also identifies the subtype, for example, influenza A H1N12009, although viral culture remains the gold standard.
Inflammatory MarkersIn pneumonias that require hospitalisation, the determination of inflammatory markers (C-reactive protein, procalcitonin) constitutes an additional orientation tool for the aetiological diagnosis as well as the stratification of the severity of CAP and in monitoring the progress of the patient.45
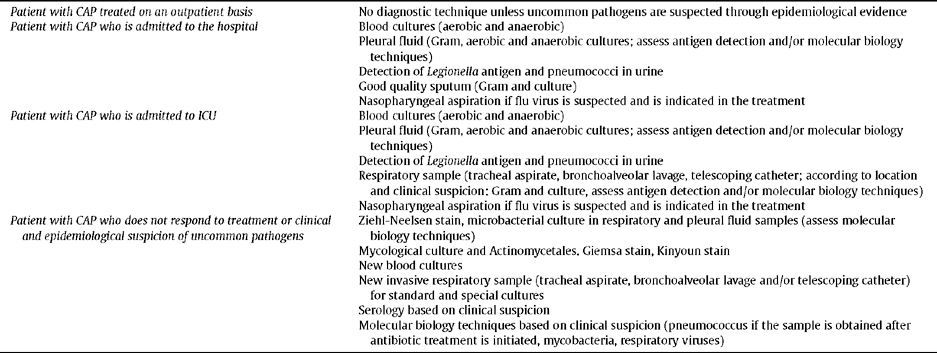
Lastly, Table 2 summarizes the applicable microbiological techniques for an aetiological diagnosis in patients with CAP.
Microbiological techniques to perform in the patient with CAP
|
|
| Patient with CAP who is admitted to ICU |
|
| Patient with CAP who does not respond to treatment or clinical and epidemiological suspicion of uncommon pathogens |
|
For the time being and until rapid diagnostic tools are available with a sensitivity and specificity of 100%, the initial treatment of CAP is empiric for the majority of patients. The outcomes of randomised prospective studies that compared the empiric antibiotic treatment versus directed treatment based on the results of rapid tests (urinary antigens for S. pneumoniae and L. pneumophila) did not demonstrate any difference in the progress of the patients.46 In general, the choice of empirical treatment is based on the microorganisms that cause CAP and the local standards for antibiotic susceptibility to these microorganisms. The decision on the type of antibiotic treatment depends upon the severity of CAP and the risk factors of the patient.21,47–48 The use of prognostic tools, such as the PSI17 and the CURB65,18 has systemised the decision to hospitalise. Although there are also criteria for ICU admission which are acceptably sensitive and specific, they are not used systematically.21 Moreover, the decision to hospitalise in an intensive care unit depends upon the resources, priorities and availability of each hospital.
Ambulatory TreatmentIn cases where patients with CAP do not require hospitalisation and can be treated on an out-patient basis, the antimicrobial treatment must cover aetiological principles, specifically, S. pneumoniae, M. pneumoniae, C. pneumoniae and L. pneumophila. It should be noted that in Spain, resistance of S. pneumoniae to macrolides is around 25%49 and there is clinical evidence of treatment failure when tested pneumococcal pneumonia is treated solely with macrolides.50 Meanwhile, although the resistance of S. pneumoniae has declined over time and the cut-off points for the minimum inhibitory concentration (MIC) are on an upward trend, it is advisable to administer high doses of penicillins or beta-lactams which enable high serum levels of antibiotics to be reached which act effectively in cases of resistance at an intermediate level.21,47–49
Given these considerations, clinical studies clearly demonstrate that the administration of a beta-lactam plus a macrolide or a quinolone alone have the same clinical efficacy.21 As a result, combination therapy should be recommended associating amoxicillin or amoxicillin with clavulanic acid plus azithromycin or clarithromycin or even levofloxacin or moxifloxacin in monotherapy. The use of an oral cephalosporin (cefditoren) would be an alternative to combine with macrolides. In previous recommendations, SEPAR49 also recommended telithromycin in monotherapy. However, this antibiotic is no longer used especially because of its liver toxicity and, therefore, it has been eliminated from the list of recommended antibiotics.
Treatment for Patients With CAP who Require Admission to a Hospital WardMost patients admitted to the hospital met appropriate criteria for hospitalisation based on PSI17 or CRB6518 scales, but there is always a percentage of patients who for various reasons were hospitalised when they could have been treated as outpatients. In the interest of the recommendations for antimicrobial treatment, all patients are considered to theoretically meet admission criteria for this group. Furthermore, there could be patients who meet the admission criteria for ICU and are hospitalised in a conventional ward. Provided that in this case patients could benefit from a more aggressive antibiotic treatment, it is advisable to administer combination antibiotics instead of monotherapy.
Clinical trials to date in this patient group have not demonstrated any differences in clinical efficacy compared to combination beta-lactam and a macrolide versus quinolone in monotherapy.21,49,51 However, published studies include few patients in the PSI Class V risk category who are those that present greater mortality and a higher percentage of non response.51 As a result, the scientific evidence in regards to the efficacy of a beta-lactam antibiotic combined with a macrolide versus quinolone is limited. Therefore, for patients with CAP who are admitted to a hospital ward, the recommended empirical treatment is as follows: a) administration of a quinolone in monotherapy (Levofloxacin or moxifloxacin per oral or intravenous route), or b) combination of third-generation cephalosporin (cefotaxime or ceftriaxone) or amoxicillin-clavulanate with a macrolide.
Treatment for Patients with CAP who Require Admission to an ICUIn general, this population represents 10% of hospitalised patients with CAP and, as in the previous case, it is not uncommon for some patients, who do not required treatment in ICU, to be admitted to intensive care. In either case, these patients should be treated according to the recommendations that refer to those who truly require ICU admission. Furthermore, the results of retrospective and prospective studies52–54 indicate that the administration of combination antibiotics, specifically, a beta-lactam with a macrolide reduces mortality. The populations studied have been largely patients with bacteremic pneumococcal pneumonia, sepsis and septic shock so that it seems to be prudent to first recommend combination beta-lactam with a macrolide. In fact, the latest guidelines of the British Thoracic Society already recommend this.48 In the only study on patients with CAP hospitalized in ICU, in which the clinical efficacy of quinolone was compared to combination antibiotics (beta-lactam and quinolone), no significant differences were demonstrated.55 In this study, however, patients with septic shock were excluded.
As a result, the empirical treatment recommendations are as follows: a) administer preferably a beta-lactam per intravenous route (those previously recommended) combined with a macrolide per the same route, and b) in cases of failure to administer macrolides, one should opt for combination beta-lactams plus quinolone per intravenous route.
Clinical Suspicion of CAP Caused by P. AeruginosaUp until now, the recommendation to treat patients with a suspected infection due to P. aeruginosa is combination antibiotics. It has been recently found that the incidence of CAP caused by P. aeruginosa is less than initially believed56,57 for the reason that many of these pneumonias are health care-associated pneumonias (HCAP). However, excluding this population, there are still patients with severe pneumonia caused by P. aeruginosa in whom, moreover, the mortality rate is higher. In patients with advanced COPD (FEV1 >30%) or with generalised bronchiectasis who have received repeated antibiotics within the last year, empirical antibiotic treatment is recommended to cover this microorganism. In addition to administering combination antibiotics, S. pneumoniae and L. pneumophila should also be covered. Combining a carbapenem (meropenem or imipenem) or piperacillin/tazobactam with levofloxacin is probably the most indicated at present although other possibilities exist.
Clinical Suspicion of CAP Caused by Methicillin-Resistant S. aureusAt present, and especially in the United States, cases of CAP were observed caused by strains of methicillin-resistant S. aureus which has the Panton-Valentine virulence factor. In general, these cases occur in younger patients who present very serious necrotising forms. This situation is uncommon in Europe and in Spain but should be considered on occasions. Coverage for S. pneumoniae and L. pneumophila should also be provided under these circumstances, so therefore, combination of linezolid or vancomycin with levofloxacin is probably the most appropriate treatment.
Clinical Suspicion of CAP Caused by Anaerobic Microorganisms and Aspiration PneumoniaIn patients with mouth sepsis and/or a history of loss of consciousness, a lung abscess or necrotising pneumonia may be the presenting forms of CAP. Anaerobic and/or gram-negative microorganisms may be the causal agents involved. Anaerobic and/or gram-negative microorganisms should be considered in cases of aspiration pneumonia from gastric contents. In all these situations, the recommendation is the empirical administration of amoxicillin with clavulanic acid at high doses, ertapenem, piperacillin-tazobactam, clindamycin or moxifloxacin. The choice of an antibiotic varies according to tolerance and availability per oral route since prolonged treatment will be required in cases of lung abscess and necrotising pneumonia.
Other Aspects of Empirical TreatmentIn regards to the administration of the first dose of antibiotics in patients with CAP, two retrospective studies58,59 suggest that the first dose administered within the first 4 to 8 h from the time the patient arrives at an emergency department decreases mortality. These findings have been confirmed in a prospective study of patients with CAP and sepsis, having observed that the mortality rate decreases especially in patients with CAP and septic shock.60 These data have stirred considerable controversy, especially in the United States where the American Society of Emergency Medicine has recommended that no further recording is needed of the time to the administration of the first dose of antibiotic.61
Our recommendation is very similar to that included in the update of the British Thoracic Society48 so that the first dose of antibiotic should be administered in the Emergency Department and before the patient is transferred to a hospital ward. In cases of outpatient visits for the first time, a first dose of oral or intramuscular antibiotics is recommended before sending them to the hospital.
In reference to the duration of antibiotic treatment, the standard guideline is 5 to 7 days. In the recommendations from the ATS/IDSA,21 situations in which treatment should be extended are as follows: persistent fever for more than 72 h, persistence in more than one clinical instability criterion, inadequate initial coverage and the onset of extrapulmonary complications such as meningitis and endocarditis.
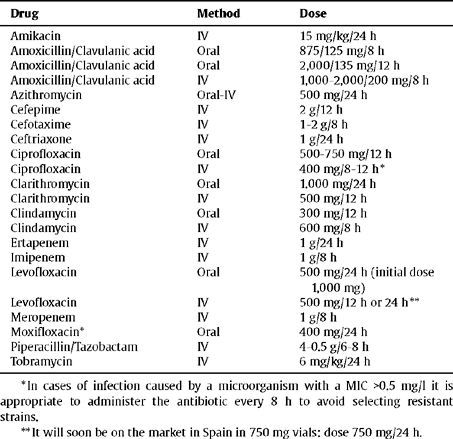
In Table 3 and Table 4 treatment guidelines are presented and the recommended doses for the main sections described in this section.
Empirical antibiotic treatment in CAP
| Out-patient treatment |
|
| Treatment when admission to a hospital ward is required |
|
| Treatment when admission to ICU is required |
|
| Suspected aspiration | Amoxicillin-clavulanic acid intravenously (amoxicillin 2 g/8 h) for 14 days or moxifloxacin, ertapenem or even clindamycin |
| Suspected infection caused by P. aeruginosa |
|
Dose and route of administration for antibiotics in CAP
| Drug | Method | Dose |
| Amikacin | IV | 15 mg/kg/24 h |
| Amoxicillin/Clavulanic acid | Oral | 875/125 mg/8 h |
| Amoxicillin/Clavulanic acid | Oral | 2,000/135 mg/12 h |
| Amoxicillin/Clavulanic acid | IV | 1,000–2,000/200 mg/8 h |
| Azithromycin | Oral-IV | 500 mg/24 h |
| Cefepime | IV | 2 g/12 h |
| Cefotaxime | IV | 1–2 g/8 h |
| Ceftriaxone | IV | 1 g/24 h |
| Ciprofloxacin | Oral | 500–750 mg/12 h |
| Ciprofloxacin | IV | 400 mg/8–12 h * |
| Clarithromycin | Oral | 1.000 mg/24 h |
| Clarithromycin | IV | 500 mg/12 h |
| Clindamycin | Oral | 300 mg/12 h |
| Clindamycin | IV | 600 mg/8 h |
| Ertapenem | IV | 1 g/24 h |
| Imipenem | IV | 1 g/8 h |
| Levofloxacin | Oral | 500 mg/24 h (initial dose 1,000 mg) |
| Levofloxacin | IV | 500 mg/12 h or 24 h ** |
| Meropenem | IV | 1 g/8 h |
| Moxifloxacin* | Oral | 400 mg/24 h |
| Piperacillin/Tazobactam | IV | 4–0.5 g/6–8 h |
| Tobramycin | IV | 6 mg/kg/24 h |
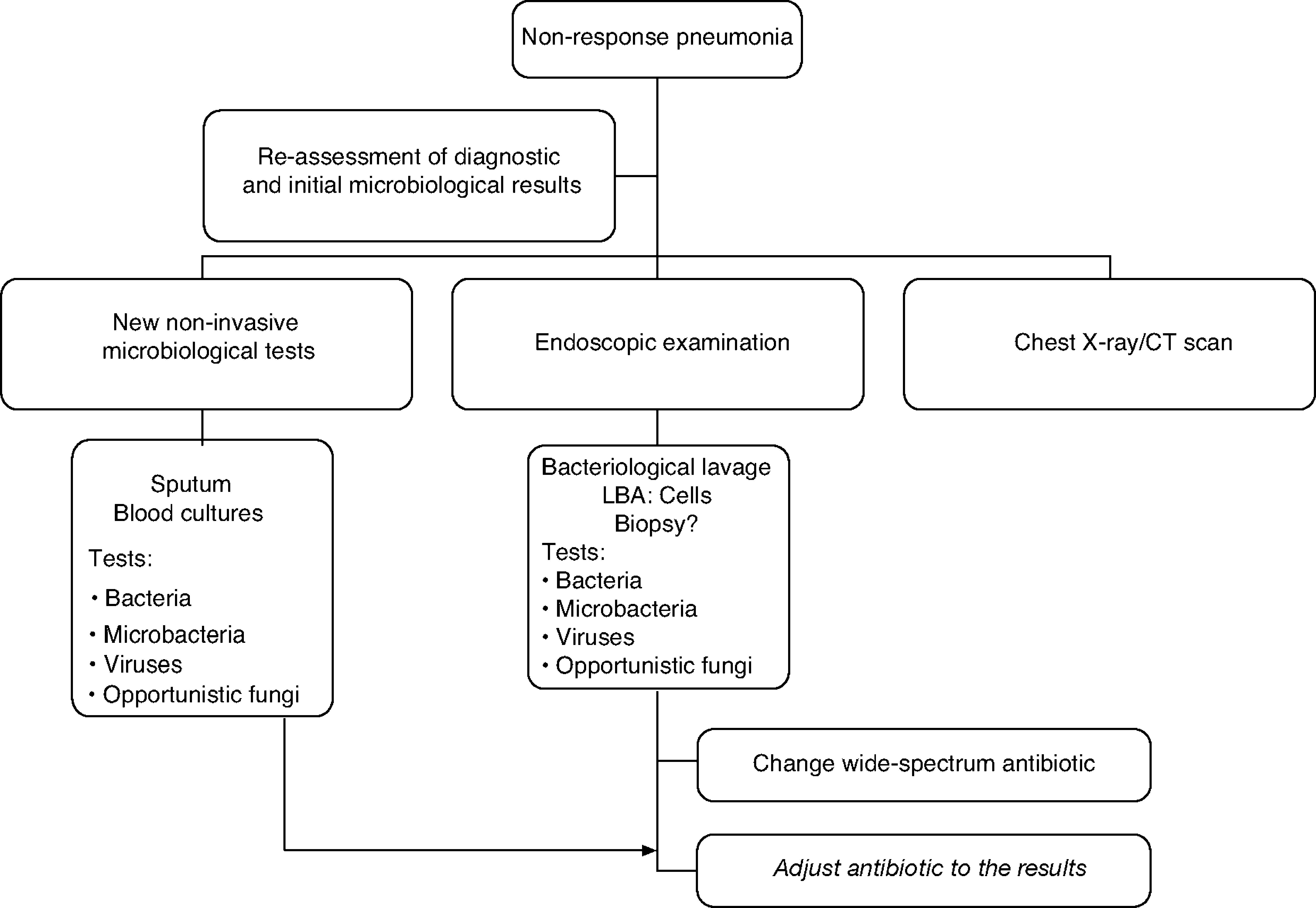
Inadequate response to antimicrobial treatment is difficult to define since it depends upon factors related to the initial severity, the causal agent and the host characteristics. Between 10 and 15% of hospitalised patients and up to 21% of ambulatory patients present unsatisfactory progress.33,62,63 Inadequate treatment response was classified according to symptoms as either a worsening or no improvement, such as the progress time of the pneumonia. As a result, non responsive CAP is defined as persistence or worsening of symptoms in the initial phase, whereas we refer to delayed resolution CAP if we are dealing with the persistence of radiological images at 4–6 weeks.
In patients with CAP treated on an outpatient basis, the need for hospitalization or a change in antibiotic treatment can be considered a non response or treatment failure.21 In hospitalised patients with CAP, two non response patterns of pneumonia have been described. The first is progressive pneumonia when there is clinical deterioration with severe respiratory failure, the need for mechanical ventilation and/or the onset of septic shock, which is more common in the first 72 h.33,63 The second is characterized by clinical instability, taking into consideration, in this case, the elapsed time until achieving clinical stability.64
The study on biomarker serum concentrations, such as C-reactive protein and procalcitonin, at 3–4 days after initiating antibiotic therapy is useful in predicting the response. If an increase or decrease in the values is observed that are 40–50% lower compared to the first day, the probability of non-response and/or the onset of complications increases. Data from the different studies demonstrate that a number of C-reactive protein < 100 mg/1 on Day 1 is a protective factor for poor outcome (odds ratio = 0.21),65 whereas values > 210 mg/ml constitute a risk factor (odds ratio = 2.6).66 When C-reactive protein concentrations are ≤30 mg/dl and procalcitonin ≤0.3 ng/ml at 72 h, there is a positive high predictive value (>95%) that no complications will arise.67
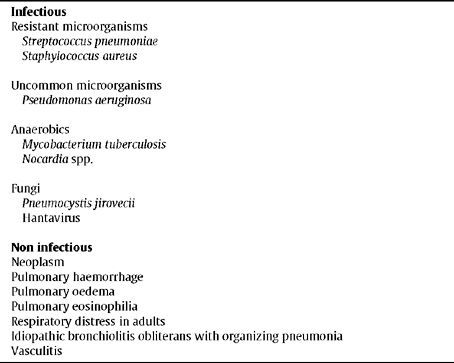
Regarding the aetiology of non responsive CAP (Table 5), 40% is infectious, 15% non-infectious and unspecified in the remainder.68,69 In a prospective cohort of 1,424 patients hospitalised with CAP, in 215 in which the outcome was unsatisfactory, the most common isolated microorganisms were S. pneumoniae, Streptococcus spp., S. aureus, L. pneumophila, M. tuberculosis, C. burnetii, P. aeruginosa and enterobacteria.70 In a study on the institutionalised elderly population, methicillin-resistant S. aureus, GNEB and P. aeruginosa were the most common microorganisms.71
Aetiology of non responsive CAP
|
|
|
|
|
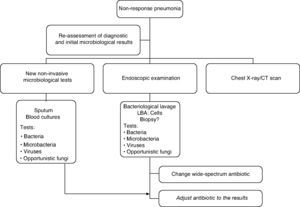
The course of action when faced with a non-responder patient includes, firstly, a complete reassessment confirming or reconsidering the diagnosis of CAP to rule out other non-infectious causes. Secondly, it is advisable to proceed with the microbiological assessment using non-invasive techniques and including invasive techniques by means of flexible bronchoscopy, supplemented by the use of other techniques such as a chest CT scan, which can be very important in determining the subsequent change in the antibiotic treatment since in 45–75% of cases the diagnosis is established through a chest CT scan. If there is no clinical deterioration and/or host characteristics (elderly, immunosuppressed patients) or microorganisms (for example: Legionella spp.) that could explain a slow response, radiographic follow-up (chest x-ray or chest CT scan which would allow the pleura and mediastinum to be examined) is a conservative option. The morphology of the infiltrates is important for the diagnostic focus and selecting the most suitable area to obtain the samples.72 Although the combination of radiological studies and taking microbiological samples by invasive and non invasive techniques successfully achieve a diagnosis in 70% of the cases,68 it has not demonstrated that it improves life expectancy.71,73
The treatment recommendation when faced with non-response is to indicate an antibiotic guideline with a microbiological spectrum much broader than the initial one and adjust it later on when the results from the microbiological studies are available. Combined treatment provides a broader spectrum and should take into consideration the initial treatment: beta-lactam anti-Pseudomonas (cefepime, imipenem, meropenem, piperacillin/tazobactam) + fluoroquinolones and assess the macrolide (azithromycin or clarithromycin). If it is an institutionalised elderly patient or if there was prior exposure to antibiotics or colonisation by S. aureus, vancomycin or linezolid should be administered until the presence of methicillin-resistant S. aureus has been ruled out.73 If there are risk factors for infection by Aspergillus spp. as in the case of patients with severe COPD, immunocompromised patients and/or those who receive systemic corticoids, antifungal treatment should be administered until this possibility has been excluded.
The treatment strategy for the non responder patient with CAP is detailed in the algorithm in Figure 1.
Prevention of CAPThe prevention of CAP can be carried out by combating the pathogens that cause them, whose prototype would be a specific vaccination against the pneumococcus, or trying to eliminate the risk situations that favour its occurrence, primarily through influenza vaccination and the fight against smoking.
Pneumococcal VaccineThe prevalence and the intrinsic virulence of the pneumococcus as well as the progressive resistance to antibiotics observed in recent decades, has rekindled interest in the development and improvement of anti-pneumococcal vaccines. Currently, there are two types: the 23-valent polysaccharide vaccine (VP-23) and heptavalent-conjugate vaccine (CV-7).
The VP-23 contains the purified capsular polysaccharides of the 23 most common serotypes, although the response is poor in the populations most at risk for severe CAP (children under 2 years old, the elderly, the immunocompromised) and irregular when faced with different serotypes. The outcomes in the studies published in the literature demonstrate the effectiveness of the VP-23 vaccine in reducing the risk of invasive pneumococcal pneumonia in immunocompetent adults74 and a better outcome in patients who develop CAP,75,76 even though it was not able to demonstrate its action to reduce the risk of non-invasive pneumococcal pneumonia.74
The VC-7, which has been successfully utilised for a decade,77,78 protects against the seven serotypes that account for 80% of pneumococcal infections (otitis media, pneumonia and meningitis) in children. In this group, this vaccine is highly immunogenic for its T-lymphocyte-dependent response and has demonstrated a significant reduction in the incidence of pneumonia and invasive pneumococcal disease in children under one year of age.77,79 Furthermore, since young children are the most important reservoir of pneumococci, the elimination of their carrier status reduces the risk of transmission to the rest of the population and thus the frequency of invasive pneumococcal infection even in unvaccinated subjects,78,80,81 which, on the other hand, forces us to rethink about the studies to date conducted on the cost-effectiveness of vaccination with VP-23 in adults. However, the long-term benefits of using the VC-7 have been challenged by the increase in invasive disease caused by serotypes of S. pneumoniae not included in the vaccine, especially serotype 19A,82 which in addition to having a more aggressive potential as a pulmonary and extrapulmonary pathogen, favours the acquisition of genes associated with multidrug resistance. These epidemiological changes warrant the need for new strategies in the design of antipneumococcal vaccines with broader protection and a greater number of serotypes. Recently, two vaccines have completed the required clinical development and have been authorised by the FDA and the EMA: the 10-valent vaccine (Synflorix®, GlaxoSmithKline) and the 13-valent vaccine (Prevenar 13®, Pfizer). The VC-10 also includes the serotypes from the VC-7, 1, 5, 7F and is used as a carrier protein for eight of the ten serotypes, protein D, a 42 kD lipoprotein obtained from the external membrane of nontypeable H. influenzae. It was approved by the EMEA and indicated in the prevention of invasive pneumococcal disease (IPD) and acute otitis media (AOM) caused by pneumococci in children between 6 weeks and 2 years of age. The VC-13 incorporates serotypes 1, 3, 5, 6A, 7F and 19A to VC-7, using the same carrier protein that is the non-toxic mutant of diphtheria toxin (CRM197). It was approved by the EMA and indicated in the prevention of invasive pneumococcal disease, pneumonia and acute otitis media caused by pneumococci in children between 6 weeks and 5 years of age. Both vaccines have demonstrated similar safety and reactogenicity to VC-7 and can be co-administered with other routine vaccination schedules without significant immune interference and without increasing reactogenicity.83,84
After its administration, the vaccine can cause mild local side effects (pain, redness or swelling) in half of the cases, which usually do not last more than 48 h. Moderate systemic reactions (fever or myalgia) or more severe local reactions (induration) are rare. The VP-23 should not be administered per intradermal route or during acute pneumococcal infection. It has not been assessed for safety during the first trimester of pregnancy or in breastfeeding. The antipneumococcal vaccine can be administered simultaneously with other vaccines like the flu, but in a different site. Regarding immunosuppressive treatment, initiation should be postponed at least 2 weeks after vaccination and is not recommended during chemotherapy or radiotherapy.
Antipneumococcal vaccine provokes a humoral response that decreases starting 5–10 years after vaccination. The administration of a first dose of VP-23 attenuates the immune response of subsequent doses85 and, consequently, decreases the clinical protection provided by re-vaccination. In a recent clinical trial, the elderly who were vaccinated with VC-7 a year after having been vaccinated with VP-23 had lower levels of antibodies and better functional opsonophagocytic activity than those who received VC-7 for the first time.86 The clinical significance of this observation is unknown. Administration of a second dose of vaccine is recommended for patients over 65 years who were vaccinated for the first time before reaching that age, provided it has been at least 5 years since they received the first dose. Currently, revaccination is recommended only in cases of asplenia and immunosuppression. If a child has received the VC-7 vaccine and is over 2 years old, the minimum interval in order to administer the VP-23 is 2 months.87 Although local reactions are more common in adults who received a second dose of VP-23, revaccination does not appear to be associated with clinically significant adverse effects.88
Recommendations for the use of VP-23 and VC-789,90 are specified in Table 6 and Table 7.
Recommendations for use of 23-valent polysaccharide vaccine
|
Recommendations for use of heptavalent-conjugate vaccine
|
In Spain, the flu epidemic occurs starting from the end of autumn until the beginning of spring. It affects 1–5% of the population and 40–50% of people over 65 years. The influenza vaccine could actually prevent the disease in 70–90% of healthy people under 65 years. In older subjects or subjects with chronic debilitating diseases, the efficiency is lower, but it can attenuate the disease and condition fewer lower respiratory tract infections as well as reduce morbidity and mortality associated with influenza infection.91,92 The efficacy of the vaccine depends upon the similarity between the circulating viral sequence and the vaccine administered as well as host factors. The influenza vaccine should be given to all persons over 6 months who do not have any contraindications (Table 8), particularly in populations with a higher risk of complications or in healthy subjects who are in close contact with persons at high risk of contracting the virus (healthcare personnel).93
Recommendations for influenza vaccine
|
There are two types of vaccines that are equally effective; the activated and inactivated vaccine. The inactivated vaccine contains dead or inactivated viruses. It is administered by intramuscular injection and can be given to all people who are 6 months or older, including both the healthy population and those with chronic diseases. The attenuated vaccine contains live attenuated virus that can replicate and spread. It is administered intranasally, is more expensive and has only been approved for the healthy population aged between 2 and 49 years, except for pregnant women, and including those in direct contact with high-risk population (except in immunocompromised patients requiring a protected environment, such as recipients of haematopoietic cell transplant). Recently, the first intradermal influenza vaccine was approved that is able to induce a potent immune response with lower doses of antigen.
The concern over potential side effects has limited its use in some patients. The inactivated vaccine does not contain any live viruses and, therefore, can not cause influenza infection, although an oculo-respiratory syndrome has been described (red eyes, facial oedema and respiratory symptoms) which is self-limiting after administration. The attenuated vaccine can provoke flu-like signs and symptoms (pharyngodynia, nasal congestion, fever, headache and myalgias) and mild local reactions that usually last for less than 24 h. People with an acute, moderate or severe febrile syndrome should not be vaccinated until the symptoms subside and special precautions should be taken for patients with hypersensitivity to eggs. The estimated risk of Guillain-Barré syndrome associated with the vaccine is low and the potential benefits from the vaccination largely outweigh the risks. However, as a measure of precaution, people who do not have a high risk for serious complications from the flu and have suffered from Guillain-Barré syndrome within the last 6 weeks or after receiving a previous vaccine should not get vaccinated.
In April 2009, a new strain of influenza, A (H1N1), was identified that is antigenically and genetically different from other human influenza A (H1N1) viruses circulating since 1977. This virus spread worldwide within a few weeks. For this reason, in June that same year, the World Health Organization declared the situation a global pandemic.94 Unlike cases of seasonal flu, the new influenza A (H1N1) entails a higher number of hospitalizations in subjects under 65 years old. Vaccination is the most effective method to prevent the disease and its complications. At present, the five population groups with a priority indication for immunisation are: pregnant women, caregivers for children less than 6 months or who live with them, health care workers, people aged between 6 months and 24 years, and people aged between 25 and 64 with diseases that may worsen or provoke complications from influenza infection.95 The number of vaccine doses required for immunisation against this new virus has not been established. Simultaneous administration, in different anatomical sites, of inactivated vaccine against seasonal influenza and the new A influenza virus is perfectly feasible. However, this practice is not recommended in cases where live attenuated viruses are used.
Other Preventive Measures Against InfluenzaThe development of an adequate immune response after the inactivated influenza vaccine may require more than two weeks in adults, therefore, in specific cases (cohabitants, workers in institutions which have detected an outbreak, or those who, within the context of an outbreak, are at high risk of complications), chemoprophylaxis may be useful. It could also be indicated for people with contraindications to receiving the vaccine or who develop a poor response to it. The antiviral drugs should not be used as adjuvant therapy to the attenuated vaccine. Frequent hand-washing and respiratory hygiene are other useful, reasonable and inexpensive non pharmacological measures to control influenza and its potential complications. Not enough information is available at present related to other community or population strategies (school closures, the use of masks) to try to mitigate the spread of influenza during seasonal epidemics.93
The Fight Against SmokingSmoking is an independent risk factor in CAP96,97 and invasive S. pneumoniae infection in young people.98 Furthermore, it increases the risk of CAP and the incidence and severity of pneumonias due to varicella and Legionella spp.99,100 The cessation of smoking decreases the risk of suffering from CAP in half within 5 years after giving up the habit. Consequently, smoking cessation should be a high priority in patients who smoke that present CAP.101
Summary of the RecommendationsEpidemiologyThe annual incidence of CAP is 5–11 per 1,000 of the adult population (Level Ib).
The incidence varies with age and is greater in the extremes of life (Level Ib).
The number of patients with CAP who require hospitalisation varies between 1.1 and 4 per 1,000 (Level Ib).
The percentage of hospitalised patients who require medical management in ICU varies between 1.2 and 10% (Level Ib).
The mortality rate reported for patients with CAP treated in the community is less than 1%, ranging between 5.7 and 14% in hospitalised patients, with about 30% being patients requiring ICU and it may reach 50% in patients requiring assisted ventilation (Level Ib).
The aetiology of CAP cannot be deduced with reliability by taking into consideration only the clinical signs and symptoms (Level II).
The most common causal agent in all categories is S. pneumoniae (Level II).
Elderly patients may often present a clinical picture that is unremarkable (Level II).
Chest x-ray is a basic test to establish the diagnosis of CAP (Level II).
Initial Assessment of the Severity and Prognostic ScalesThe severity assessment is a priority and enables decision-making as to whether the patient should be hospitalised or treated as an outpatient (Level IVB).
The severity assessment is based primarily on the clinical judgement of the attending physician who will use prognostic scales as an additional support tool. In making the decision for admission, in addition to the severity, other aspects must be taken into consideration such as the degree of stability of potential co-morbidities and the social circumstances of the patient (Level IVB).
At a hospital level, the PSI or the CURB 65 may be used as prognostic scales. Hospital admission is recommended for PSI risk classes III or higher, or 2 or more points on the CURB65 scale. The patients who belong to PSI risk classes IV to V or with 3 or more points on the CURB65 scale should be treated as severe cases (Level II).
The CURB scale 65 is used at the community level. The patients with 0 points can be treated at home. Starting from 1 point, the severity progressively rises and hospital referral should be considered. Transfer to the hospital must take place urgently with a score of 3 or 4 points (Level II).
Microbiological DiagnosisIn patients with CAP treated as outpatients, there is no need to perform any diagnostic test, unless uncommon pathogens are suspected due to epidemiological evidence (Level III).
In patients with CAP admitted to hospital the performing of blood cultures (aerobes and anaerobes) and pleural fluid culture are indicated, as well the detection of Legionella antigen and pneumococcus antigen in urine, and obtaining a good quality sputum sample. If influenza virus is suspected and antiviral treatment is indicated, a nasopharyngeal aspirate is required.
In patients with CAP admitted to ICU, besides the previously mentioned samples, the obtaining of a respiratory sample would be indicated, using an invasive technique (tracheal aspirate, bronchoalveolar lavage, telescopic catheter; depending on location and clinical suspicion), to perform a Gram stain and culture, as well as to evaluate antigen detection and/or molecular biology techniques (Level III).
In patients with CAP who do not respond to treatment or a clinical and epidemiological suspicion of uncommon pathogens, it would be advisable to perform special stains and cultures to look for mycobacteria, fungi and actinomycetes in respiratory samples and pleural fluid. It would also be advisable to obtain new blood cultures and a new respiratory sample using an invasive technique for conventional and special cultures and performing molecular biology techniques depending on clinical suspicion. To look for uncommon pathogens, serology depending on clinical suspicion (Level IV).
Antimicrobial TreatmentStratify the patients into three groups for empirical treatment: a) those that can be treated on an outpatient basis; b) those that should be treated on a conventional hospital ward, and c) those who are admitted to ICU (Level I).
Initiate empirical treatment as soon as possible for both outpatients in the Emergency Department and especially patients with greater severity (Level II).
Outpatients: amoxicillin or amoxicillin/clavulanate or cefditoren plus azithromycin or clarithromycin (oral route) or even levofloxacin or moxifloxacin in monotherapy (oral route) (Level I).
Patients admitted to the ward: ceftriaxone or cefotaxime (IV route) plus azithromycin or clarithromycin, or even levofloxacin (IV or oral route) or moxifloxacin (oral route) (Level I).
In patients who meet ICU criteria but are admitted to a ward, it is advisable to use the treatment recommendations for ICU patients (Level I).
Patients admitted to ICU: ceftriaxone or cefotaxime plus azithromycin or clarithromycin intravenously as the first option. The alternative is to combine ceftriaxone or cefotaxime with levofloxacin or moxifloxacin (Level IV).
In cases where P. aeruginosa is highly suspected, combination meropenem or imipenem or piperacillin-tazobactam with levofloxacin is recommended (Level IV).
If methicillin-resistant S. aureus is highly suspected, the administration of linezolid or vancomycin is recommended (Level IV).
If necrotising pneumonia or lung abscess is present, amoxicillin with clavulanic acid at high doses, ertapenem or clindamycin can be administered (Level II).
The general duration of antibiotic treatment will be between 5 to 7 days depending upon the severity of the CAP. More prolonged antibiotic treatments are considered depending upon different factors (Level II).
CAP That Does not Respond to TreatmentThe course of action when faced with a non-responder patient includes a complete reassessment (Level II).
Microbiological assessment with non-invasive techniques and even invasive techniques jointly with other studies (chest CT scan) are vital in establishing the diagnosis and to indicate a change in antimicrobial treatment (Level II).
The treatment recommendation when faced with non-response is to indicate an antibiotic guideline with a microbiological spectrum much broader than the initial one and adjust it later on when the results from the microbiological studies are available (Level IV).
Combined therapy provides a broader spectrum and should take into consideration the initial treatment: beta-lactam anti-Pseudomonas (cefepime, imipenem, meropenem, piperacillin/tazobactam) + fluoroquinolones and assess the macrolide (azithromycin or clarithromycin) (Level III).
If it is an institutionalized elderly patient or if there was prior exposure to antibiotics or colonization by S. aureus, include vancomycin or linezolid until the presence of methicillin-resistant S. aureus can be ruled out.
In patients with risk factors for Aspergillus spp. infection, antifungal treatment should be administered until this infection can be ruled out (Level IV).
PreventionAll people who are at high-risk for pneumococcal infection or if the infection is severe or presents complications, should be vaccinated against pneumococci (Level II-III).
Vaccination is recommended in immunocompromised patients (including HIV), those diagnosed with congenital immunodeficiency, lymphoma, Hodgkin's disease, leukaemia, multiple myeloma, disseminated neoplasms, transplanted patients receiving immunosuppressive therapy, including systemic steroids, and those with nephrotic syndrome chronic renal failure on dialysis (Level II-III).
Re-vaccination is not recommended except in cases of asplenia and immunosuppression (Level III).
The influenza vaccine should be given to all persons over 6 months who do not have any contraindications with special emphasis placed on populations with a higher risk of presenting complications or in healthy subjects who are in close contact with people at high risk of contracting the virus (Level I).
Smokers who present CAP should stop smoking (Level I).
Dr. Marta Pulido for correcting the manuscript.