A common complaint in patients is chronic cough (CC), which may be refractory (RCC) or unexplained (UCC). Recent studies point, as a possible cause of CC, to the hereditary cerebellar ataxia with neuropathy and bilateral vestibular areflexia syndrome (CANVAS), with an estimated carrier prevalence of 1 in 20000.
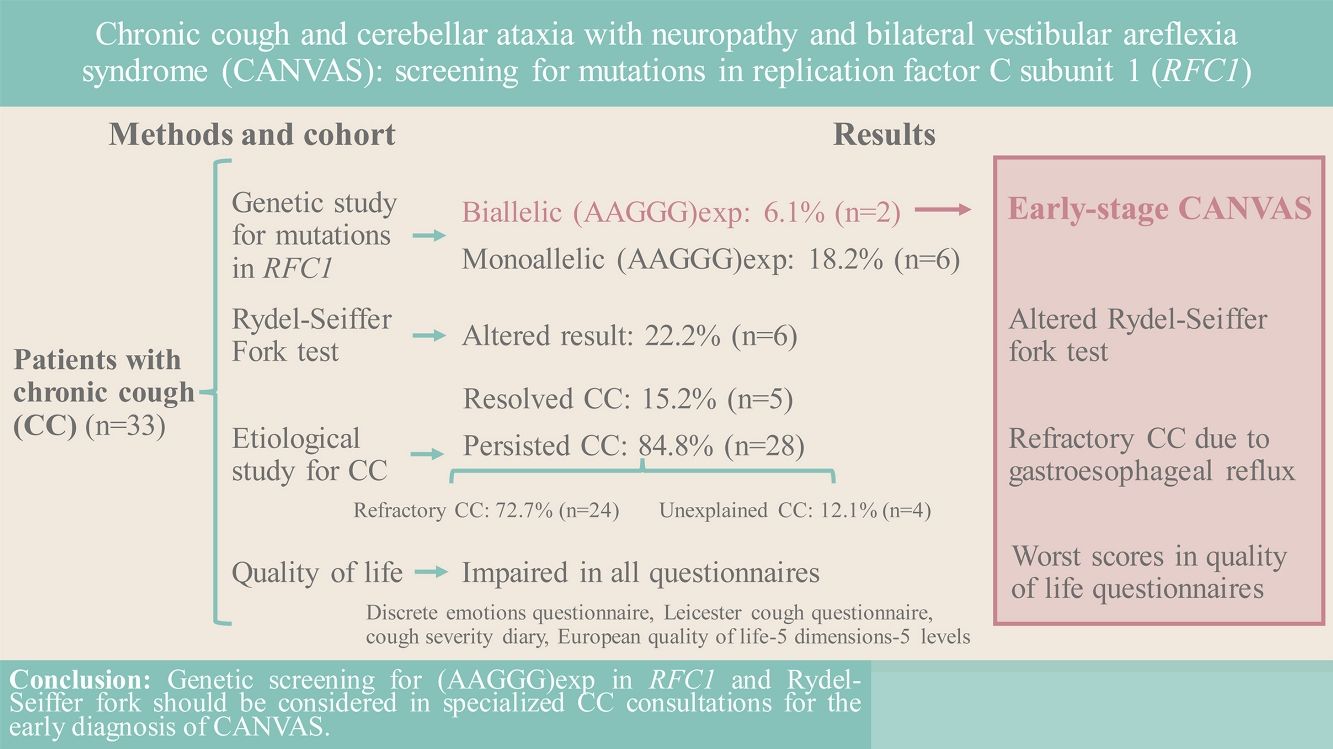
AimIn patients with CC, determine the prevalence of the biallelic (AAGGG)exp mutation in replication factor C subunit 1 (RFC1) responsible for CANVAS, test the usefulness of the Rydel-Seiffer fork test, and evaluate patient quality of life (QoL).
MethodsClinical and functional data were collected for the 33 included patients undergoing CC studies in our specialized unit. Performed were an etiological study of CC following European Respiratory Society recommendations, a genetic study of RFC1 mutations, and Rydel-Seiffer fork testing to detect possible peripheral vibratory sensitivity impairment. Administered to evaluate QoL were 4 questionnaires.
ResultsPrevalence of biallelic (AAGGG)exp in RFC1 was 6.1% (n=2) overall, increasing to 7.1% in the RCC subgroup, and to 33.3% in the Rydel-Seiffer fork altered results subgroup. Prevalence of monoallelic (AAGGG)exp in RFC1 was 18.2% (n=6) overall, rising to 50.0% (n=2) in the UCC subgroup.
ConclusionGenetic screening for (AAGGG)exp in RFC1, and also use of the Rydel-Seiffer fork test, should be considered in specialized CC consultations for patients with RCC and UCC. Detecting possible CANVAS symptoms in CC studies would identify candidates for early genetic screening, of interest in reducing the disease burden for patients and health systems alike.
Chronic cough (CC), defined as cough lasting >8 weeks,1 affects 3–12% of the general population2,3 and accounts for around 10% of pulmonology consultations.4 When the cause is not identified after a targeted study, it is classified as unexplained CC (UCC), and when the CC not respond to treatment of possible underlying cause, it is classified as refractory CC (RCC).5 Recently, the European Respiratory Society (ERS) Task Force proposed a new term to encompass both CC types, namely, cough hypersensitivity syndrome,5,6 clinically reflecting CC as an irritative cough that is frequently triggered by stimuli that do not normally produce cough.
A number of neurological diseases have been associated with CC, including Holmes-Adie syndrome7 and sensory neuropathy type 1B,8 although the underlying mechanism is still unknown. Recently, a rare disease called cerebellar ataxia with neuropathy and bilateral vestibular areflexia syndrome (CANVAS) has been postulated as a possible cause of CC, given that CC is highly prevalent (30–97%) in patients with CANVAS.9–12 The most widely accepted hypothesis regarding the underlying mechanism of CC is an alteration of the sensory innervation of the C fibres in both the upper respiratory tract and oesophagus, leading to the hypersensitivity that favours the development of CC.9,13,14 Various studies of patients with CANVAS also report gastroesophageal reflux (GER),9,13,15–17 suggesting that early alteration of esophageal motility may be the possible cause of GER and, in turn, of CC.
The relevance of CC for patients with CANVAS is that CC seems to precede the onset of neurological symptoms by years, and even decades.13,15,18 Therefore, given the possibility of early diagnosis of a progressively disabling neurological syndrome, genetic screening and counselling is important. The genetic cause of CANVAS, an hereditary autosomal recessive disease is, in most cases, the presence of biallelic expansion of the AAGGG repeat motif (AAGGG)exp in intron 2 of replication factor C subunit 1 (RFC1).10 It has also been reported that CANVAS may exceptionally be due to (AAGGG)exp and a truncating variant (nonsense or frameshift),19–21 or (AAAGG)exp when repeats are >500.22 Further alleles involved have also recently been identified (AGGGC, AAGGC and AGAGG).22 Early diagnosis is clearly important in terms of improving quality of life (QoL) for the patient and reducing the CC burden for the health system.23,24
While the precise prevalence of CANVAS is unknown, allelic frequency in (AAGGG)exp in RFC1 is estimated at 0.7% of the healthy population.10 Prevalence of biallelic carriers is therefore estimated as approximately 1 in 20000 births,10 and, since CC is a common symptom of CANVAS, a higher prevalence would be expected in those patients. In fact, that was what was observed in the study by Guilleminault et al.,25 which is, to our knowledge, the only study to date that has analyzed repeat expansion in RFC1 in patients with RCC; those authors found that 16.2% and 8.8% of patients had biallelic and monoallelic expansion, respectively. Subsequent publications raise the possibility of including genetic screening as part of RCC and UCC studies12,26; however, more research is needed to replicate and expand on available results, including, for example, for patients with respiratory pathologies26 and patients with CC undergoing a protocolized study.
This study is one of the first screens for RFC1 mutations in patients with CC describing patient clinical and functional characteristics and evaluate their QoL. Furthermore, it is the first to evaluate use of the Rydel-Seiffer fork in patients with CC to detect possible peripheral vibratory sensitivity impairment, which could be a warning sign to suspect CANVAS.
MethodsStudy design and populationCross-sectional descriptive study that included consecutively recruited patients of both sexes aged 40–90, and undergoing CC studies in a specialized hospital unit in Spain. CC was defined as cough lasting >8 weeks. Excluded were active smokers and patients who had given up smoking in the previous 12 months, patients taking angiotensin-converting enzyme inhibitors, and patients with a confirmed CANVAS diagnosis or with neurological symptoms indicative of CANVAS.
MethodologyGenetic study of biallelic (AAGGG)exp in RFC1A peripheral blood sample was obtained from all patients for a genetic study of RFC1 mutations, differentiating between monoallelic and biallelic repeat motifs of (AAAAG)exp, (AAAGG)exp, and (AAGGG)exp. DNA underwent two amplification procedures: (1) amplification by standard polymerase chain reaction (PCR) with primers flanking the intron 2 fragment of the RFC1 gene (flanking PCR), and (2) amplification by repeat-primed (RP) PCR in 3 independent reactions, namely, RP-PCR1 to detect the (AAAAG)11 and (AAAAG)exp alleles, RP-PCR2 to detect the (AAAGG)exp allele, and RP-PCR3 to detect the pathological (AAGGG)exp allele. Note that flanking PCR can only amplify alleles of normal size, not alleles that contain large expansions, regardless of their composition. The reference sequence used was NM_002913.4.
Rydel-Seiffer forkVibratory sensitivity has traditionally been studied using the standard tuning fork, which was adapted by Rydel and Seiffer in 1903 as an 8-point quantitative scale.27 In our study, patients were tested by placing the Rydel-Seiffer fork on the first metatarsophalangeal joint. Patients with a result of 6 or less – considered impairment (the patient has less peripheral vibration sensitivity than expected) – were referred to neurology.
Aetiology studyThe aetiology study was carried out following ERS recommendations.28 All patients underwent thoracic imaging, radiography or computed tomography (CT). Spirometry was performed (Datapir-600 device; Sibelmed SA, Barcelona, Spain) following American Thoracic Society (ATS)/ERS recommendations.29,30 Bronchodilator tests were considered positive at >10% of predicted forced expiratory volume in the first second (FEV1) or forced vital capacity (FVC) value 15min after administration of 4×100-μg doses of salbutamol.30 Fraction of exhaled nitric oxide (FENO) was measured (N-6008 chemiluminescence sensor; SIR, Madrid, Spain) following ATS/ERS recommendations.31 A pneumoallergens prick test, performed with standardized extracts, was considered positive for papules>3mm. Peripheral eosinophils were measured by automatic cell counting (Coulter Max-M), and immunoglobulin E (IgE) was determined by enzyme immunoassay (UNICAP, Pharmacia, Uppsala, Sweden).
Esophageal 24-h pH was measured (Digitrapper Recorder system; Medtronic, Minneapolis, USA) from a pH-impedance probe or a 1-electrode probe placed at a distance of 5cm from the upper margin of the lower esophageal sphincter, and analyzed results (Reflux software V.6.1; Medtronic, Minneapolis, USA) were interpreted following Lyon Consensus recommendations.32 High-resolution esophageal manometry data (ManoScan system; Medtronic, Minneapolis, USA) were analyzed (ManoView ESO software V.3.3; Medtronic, Minneapolis, USA) for interpretation following the Chicago Classification V.4.0.33
QoL impactTo determine the QoL impact of CC, patients were administered the Discrete Emotions Questionnaire (DEQ),34 Leicester Cough Questionnaire (LCQ),35,36 Cough Severity Diary (CSD),37 and European Quality of Life-5 Dimensions-5 Levels (EQ-5D-5L).38,39
- (1)
DEQ consists of 32 questions regarding emotions grouped into 8 states (anger, disgust, fear, anxiety, sadness, desire, relaxation, and happiness), scored on a 7-point Likert scale (1=lowest intensity to 7=highest intensity).
- (2)
LCQ consists of 19 questions on cough impact in the previous 2 weeks grouped into 3 domains (physical, psychological, and social), scored on a 7-point Likert scale (1=least impact to 7=greatest impact), with the overall score obtained by summing the domain scores.
- (3)
CSD consists of 7 questions on cough severity in the previous 24h grouped according to 3 dimensions (frequency, intensity, and disruptiveness), scored on an 11-point Likert scale (0=lowest severity to 10=greatest severity).
- (4)
EQ-5D-5L part 1 consists of 5 questions on 5 QoL domains (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression) with 5 response options, from no problems to extreme problems (variable depending on the reference population, in our case Spanish38), resulting in a health state index (where 0 is equivalent to death) scored between <0 (worse than death and 1 (full health). EQ-5D-5L part 2 is a visual analogue scale (VAS) which the patient uses to rate their perceived health (0=worst imaginable health to 100=best imaginable health).
The study complies with the principles of the Declaration of Helsinki (18th World Medical Assembly, 1964) and was approved by the Ethics Committee of our hospital (IIBSP-TOS-2020-143) and has been registered with clinicaltrials.gov (NCT04703595).
Statistical analysisDescriptive reference values are reported as frequencies and percentages for qualitative data, and as means and standard deviation (SD) for quantitative data. For the qualitative variables, independent proportions were compared using Pearson's Chi-square or Fisher's exact test; for quantitative variables, given that one of the groups included <10 patients, means were compared using the non-parametric Mann–Whitney U test.
Statistical significance was set to 5% (α=0.05), and statistical analyses were performed with SPSS version 25 (SPSS Inc., Chicago, IL, USA).
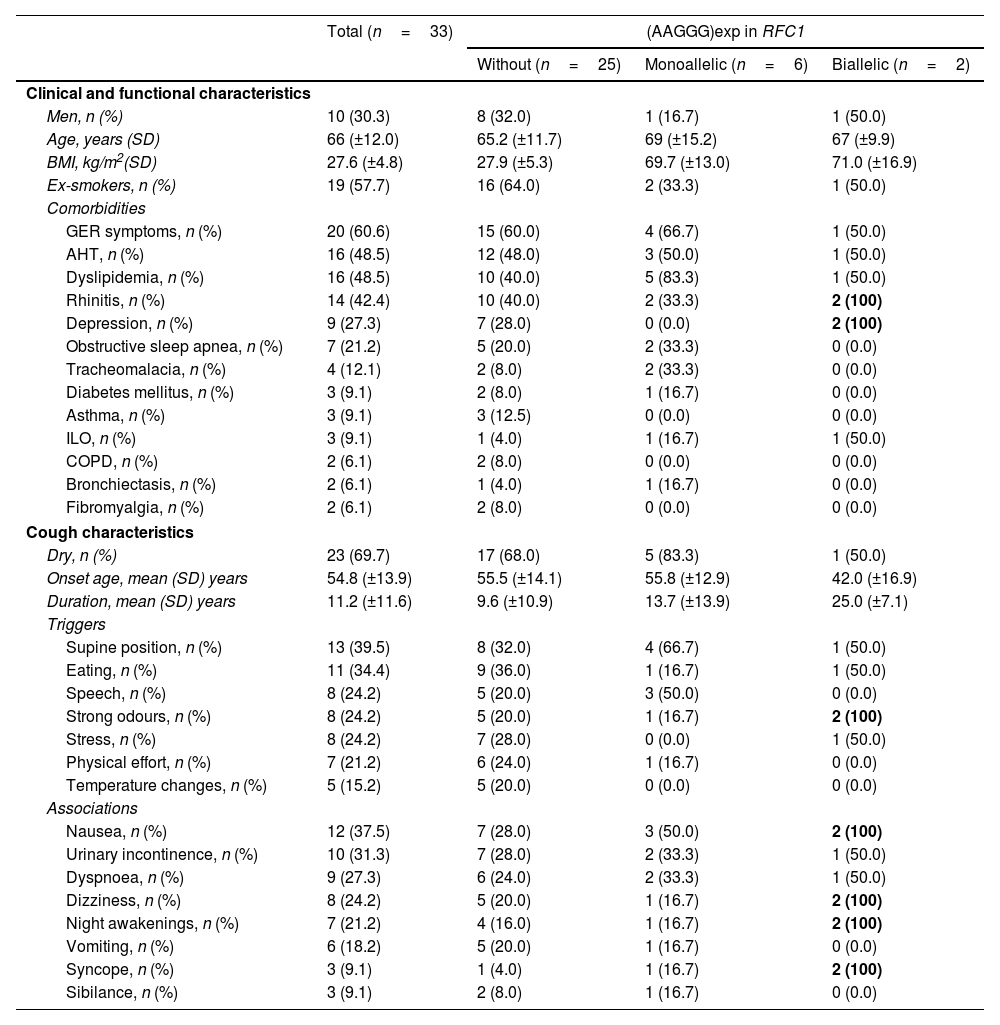
ResultsPatient clinical and functional characteristicsIncluded were 33 patients (Table 1), 30.3% (n=10) men, with a mean age of 66 (±12) years; 57.7% (n=19) were ex-smokers since an average of 25.3 (±12.9) years previously. The most frequent comorbidities were symptoms compatible with GER (60.6%; n=20) and arterial hypertension (AHT) (48.5%; n=16). CC was dry in 69.7% (n=23) of the patients. Overall, mean CC duration was 11.2 (±11.6) years and mean age at CC onset was 54.8 (±13.9) years. In the biallelic (AAGGG)exp subgroup, mean CC duration was longer and mean age at CC onset was lower, at 25.0 (±7.1) years and 42.0 (±16.9) years, respectively. No statistically significant differences were found between patients with and without (AAGGG)exp in RFC1 (Table 1).
Clinical and functional characteristics of patients with chronic cough (CC) without and with biallelic or monoallelic (AAGGG)exp in RFC1.
| Total (n=33) | (AAGGG)exp in RFC1 | |||
|---|---|---|---|---|
| Without (n=25) | Monoallelic (n=6) | Biallelic (n=2) | ||
| Clinical and functional characteristics | ||||
| Men, n (%) | 10 (30.3) | 8 (32.0) | 1 (16.7) | 1 (50.0) |
| Age, years (SD) | 66 (±12.0) | 65.2 (±11.7) | 69 (±15.2) | 67 (±9.9) |
| BMI, kg/m2(SD) | 27.6 (±4.8) | 27.9 (±5.3) | 69.7 (±13.0) | 71.0 (±16.9) |
| Ex-smokers, n (%) | 19 (57.7) | 16 (64.0) | 2 (33.3) | 1 (50.0) |
| Comorbidities | ||||
| GER symptoms, n (%) | 20 (60.6) | 15 (60.0) | 4 (66.7) | 1 (50.0) |
| AHT, n (%) | 16 (48.5) | 12 (48.0) | 3 (50.0) | 1 (50.0) |
| Dyslipidemia, n (%) | 16 (48.5) | 10 (40.0) | 5 (83.3) | 1 (50.0) |
| Rhinitis, n (%) | 14 (42.4) | 10 (40.0) | 2 (33.3) | 2 (100) |
| Depression, n (%) | 9 (27.3) | 7 (28.0) | 0 (0.0) | 2 (100) |
| Obstructive sleep apnea, n (%) | 7 (21.2) | 5 (20.0) | 2 (33.3) | 0 (0.0) |
| Tracheomalacia, n (%) | 4 (12.1) | 2 (8.0) | 2 (33.3) | 0 (0.0) |
| Diabetes mellitus, n (%) | 3 (9.1) | 2 (8.0) | 1 (16.7) | 0 (0.0) |
| Asthma, n (%) | 3 (9.1) | 3 (12.5) | 0 (0.0) | 0 (0.0) |
| ILO, n (%) | 3 (9.1) | 1 (4.0) | 1 (16.7) | 1 (50.0) |
| COPD, n (%) | 2 (6.1) | 2 (8.0) | 0 (0.0) | 0 (0.0) |
| Bronchiectasis, n (%) | 2 (6.1) | 1 (4.0) | 1 (16.7) | 0 (0.0) |
| Fibromyalgia, n (%) | 2 (6.1) | 2 (8.0) | 0 (0.0) | 0 (0.0) |
| Cough characteristics | ||||
| Dry, n (%) | 23 (69.7) | 17 (68.0) | 5 (83.3) | 1 (50.0) |
| Onset age, mean (SD) years | 54.8 (±13.9) | 55.5 (±14.1) | 55.8 (±12.9) | 42.0 (±16.9) |
| Duration, mean (SD) years | 11.2 (±11.6) | 9.6 (±10.9) | 13.7 (±13.9) | 25.0 (±7.1) |
| Triggers | ||||
| Supine position, n (%) | 13 (39.5) | 8 (32.0) | 4 (66.7) | 1 (50.0) |
| Eating, n (%) | 11 (34.4) | 9 (36.0) | 1 (16.7) | 1 (50.0) |
| Speech, n (%) | 8 (24.2) | 5 (20.0) | 3 (50.0) | 0 (0.0) |
| Strong odours, n (%) | 8 (24.2) | 5 (20.0) | 1 (16.7) | 2 (100) |
| Stress, n (%) | 8 (24.2) | 7 (28.0) | 0 (0.0) | 1 (50.0) |
| Physical effort, n (%) | 7 (21.2) | 6 (24.0) | 1 (16.7) | 0 (0.0) |
| Temperature changes, n (%) | 5 (15.2) | 5 (20.0) | 0 (0.0) | 0 (0.0) |
| Associations | ||||
| Nausea, n (%) | 12 (37.5) | 7 (28.0) | 3 (50.0) | 2 (100) |
| Urinary incontinence, n (%) | 10 (31.3) | 7 (28.0) | 2 (33.3) | 1 (50.0) |
| Dyspnoea, n (%) | 9 (27.3) | 6 (24.0) | 2 (33.3) | 1 (50.0) |
| Dizziness, n (%) | 8 (24.2) | 5 (20.0) | 1 (16.7) | 2 (100) |
| Night awakenings, n (%) | 7 (21.2) | 4 (16.0) | 1 (16.7) | 2 (100) |
| Vomiting, n (%) | 6 (18.2) | 5 (20.0) | 1 (16.7) | 0 (0.0) |
| Syncope, n (%) | 3 (9.1) | 1 (4.0) | 1 (16.7) | 2 (100) |
| Sibilance, n (%) | 3 (9.1) | 2 (8.0) | 1 (16.7) | 0 (0.0) |
Abbreviations: AHT: arterial hypertension; BMI: body mass index; COPD: chronic obstructive pulmonary disease; GER: gastroesophageal reflux; ILO: inducible laryngeal obstruction; SD, standard deviation.
* Bold signals when the 2 patients with biallelic (AAGGG)exp have the indicated clinical or functional characteristic.
Of the 33 included patients, 2 (6.1%) had biallelic (AAGGG)exp in RFC1, indicating early-stage CANVAS, i.e., although their only symptom currently is CC, in the future they will develop other neurological symptoms. Another 6 (18.2%) were heterozygous carriers of (AAGGG)exp, the significance of which is as yet unknown; however, 1 heterozygous carrier had the combination (AAAGG)exp/(AAGGG)exp, which has recently been described as potentially causing CANVAS if the (AAAGG)exp motif is very large (>500 repeats).22
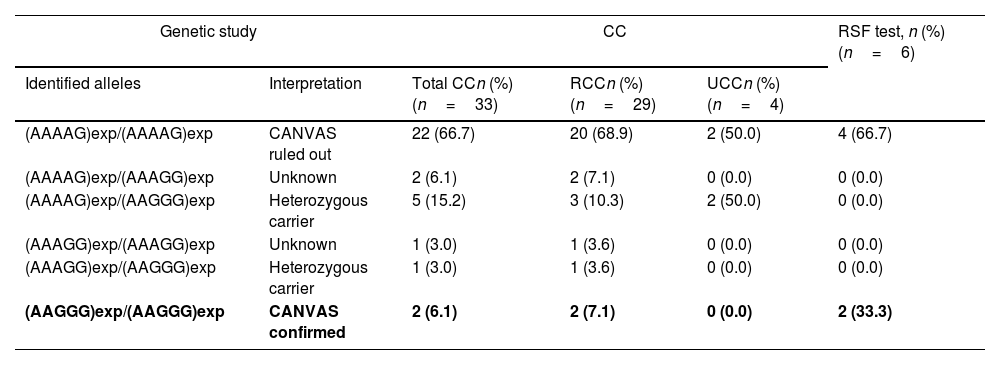
The Rydel-Seiffer fork test result was altered in 22.2% (n=6) of the patients, and of those, 33.3% (n=2) were the patients with early-stage CANVAS (Table 2).
Alleles identified in patients with chronic cough, refractory chronic cough, and unexplained chronic cough, and altered peripheral vibratory sensitivity results for the Rydel-Seiffer fork test.
| Genetic study | CC | RSF test, n (%) (n=6) | |||
|---|---|---|---|---|---|
| Identified alleles | Interpretation | Total CCn (%) (n=33) | RCCn (%) (n=29) | UCCn (%) (n=4) | |
| (AAAAG)exp/(AAAAG)exp | CANVAS ruled out | 22 (66.7) | 20 (68.9) | 2 (50.0) | 4 (66.7) |
| (AAAAG)exp/(AAAGG)exp | Unknown | 2 (6.1) | 2 (7.1) | 0 (0.0) | 0 (0.0) |
| (AAAAG)exp/(AAGGG)exp | Heterozygous carrier | 5 (15.2) | 3 (10.3) | 2 (50.0) | 0 (0.0) |
| (AAAGG)exp/(AAAGG)exp | Unknown | 1 (3.0) | 1 (3.6) | 0 (0.0) | 0 (0.0) |
| (AAAGG)exp/(AAGGG)exp | Heterozygous carrier | 1 (3.0) | 1 (3.6) | 0 (0.0) | 0 (0.0) |
| (AAGGG)exp/(AAGGG)exp | CANVAS confirmed | 2 (6.1) | 2 (7.1) | 0 (0.0) | 2 (33.3) |
Abbreviations: CC: chronic cough; RCC: refractory chronic cough; RSF: Rydel-Seiffer fork; UCC: unexplained chronic cough. *In bold, genetic results compatible with early CANVAS.
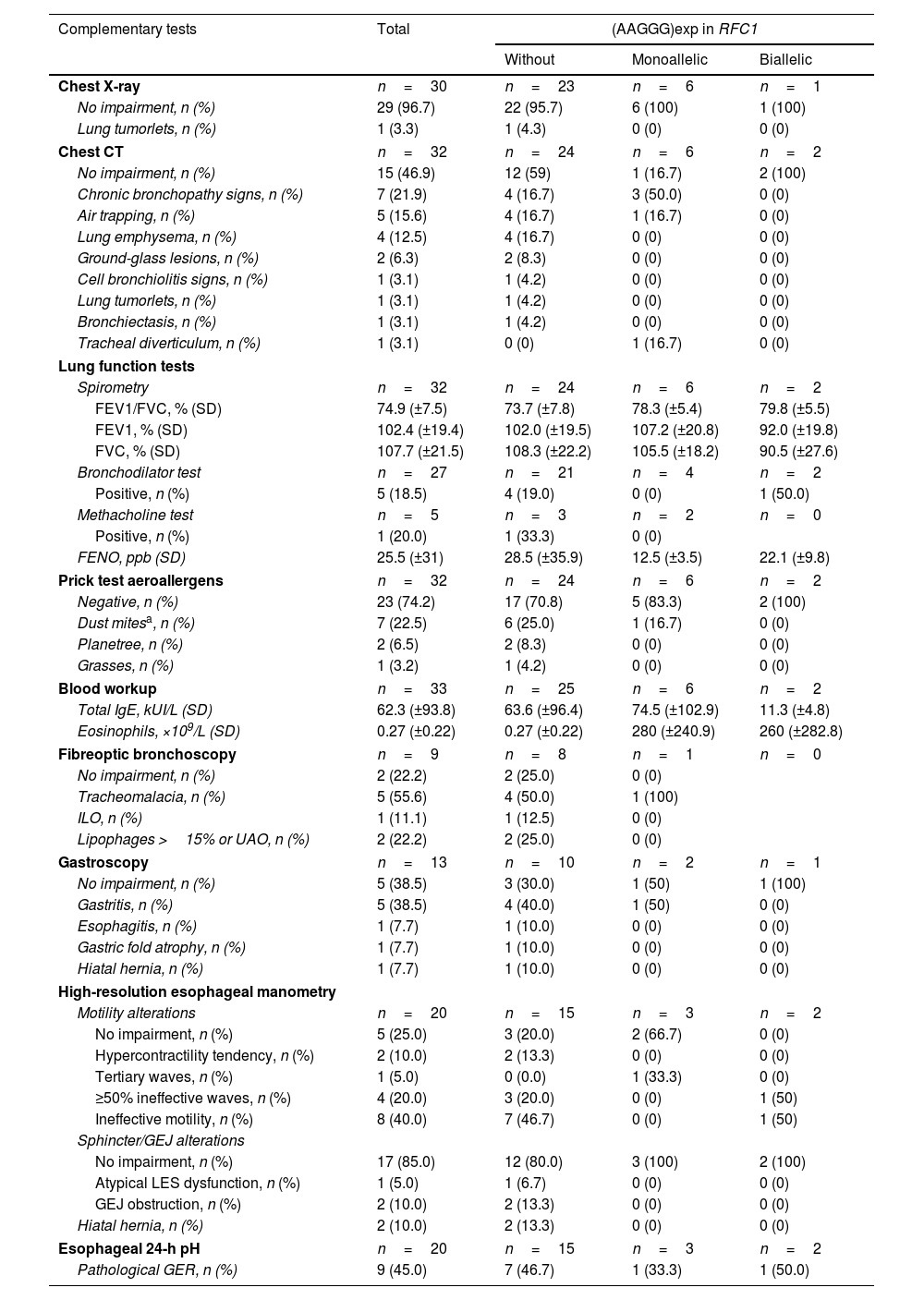
No chest X-ray alterations were evident in most patients (96.7%, n=29); detected in 1 patient were known and oncologically monitored pulmonary nodules diagnosed as tumourlets. Chest CT was normal in 46.9% (n=15) of the patients, and in the remaining patients, the most frequent alterations were chronic bronchopathy (18.8%; n=6), air trapping (15.6%; n=5), and emphysema (12.5%; n=4). Mean spirometry values were within the normal range; when studied case by case, only 1 patient (3.0%) showed obstruction, which was moderate (FEV1/FVC, 61; FEV1, 67%; FVC, 79%). Mean FENO was 25.5 (±31)ppb, and was only significantly elevated in 1 patient (133ppb). The prick test was positive in 25.8% (n=8) of patients. High-resolution esophageal manometry, performed in 20 of 33 patients, revealed motility impairment in 75% (n=15), most frequently, ineffective esophageal motility (40%; n=8) and ≥50% ineffective waves (20%; n=4). Esophageal 24-h pH monitoring revealed pathological GER in 45% (n=9) of the patients. Statistically significant differences were only found in the chest CT results on comparing the groups with and without (AAGGG)exp in RFC1 (p=0.03) (Table 3).
Chronic cough: etiological study results for complementary tests.
| Complementary tests | Total | (AAGGG)exp in RFC1 | ||
|---|---|---|---|---|
| Without | Monoallelic | Biallelic | ||
| Chest X-ray | n=30 | n=23 | n=6 | n=1 |
| No impairment, n (%) | 29 (96.7) | 22 (95.7) | 6 (100) | 1 (100) |
| Lung tumorlets, n (%) | 1 (3.3) | 1 (4.3) | 0 (0) | 0 (0) |
| Chest CT | n=32 | n=24 | n=6 | n=2 |
| No impairment, n (%) | 15 (46.9) | 12 (59) | 1 (16.7) | 2 (100) |
| Chronic bronchopathy signs, n (%) | 7 (21.9) | 4 (16.7) | 3 (50.0) | 0 (0) |
| Air trapping, n (%) | 5 (15.6) | 4 (16.7) | 1 (16.7) | 0 (0) |
| Lung emphysema, n (%) | 4 (12.5) | 4 (16.7) | 0 (0) | 0 (0) |
| Ground-glass lesions, n (%) | 2 (6.3) | 2 (8.3) | 0 (0) | 0 (0) |
| Cell bronchiolitis signs, n (%) | 1 (3.1) | 1 (4.2) | 0 (0) | 0 (0) |
| Lung tumorlets, n (%) | 1 (3.1) | 1 (4.2) | 0 (0) | 0 (0) |
| Bronchiectasis, n (%) | 1 (3.1) | 1 (4.2) | 0 (0) | 0 (0) |
| Tracheal diverticulum, n (%) | 1 (3.1) | 0 (0) | 1 (16.7) | 0 (0) |
| Lung function tests | ||||
| Spirometry | n=32 | n=24 | n=6 | n=2 |
| FEV1/FVC, % (SD) | 74.9 (±7.5) | 73.7 (±7.8) | 78.3 (±5.4) | 79.8 (±5.5) |
| FEV1, % (SD) | 102.4 (±19.4) | 102.0 (±19.5) | 107.2 (±20.8) | 92.0 (±19.8) |
| FVC, % (SD) | 107.7 (±21.5) | 108.3 (±22.2) | 105.5 (±18.2) | 90.5 (±27.6) |
| Bronchodilator test | n=27 | n=21 | n=4 | n=2 |
| Positive, n (%) | 5 (18.5) | 4 (19.0) | 0 (0) | 1 (50.0) |
| Methacholine test | n=5 | n=3 | n=2 | n=0 |
| Positive, n (%) | 1 (20.0) | 1 (33.3) | 0 (0) | |
| FENO, ppb (SD) | 25.5 (±31) | 28.5 (±35.9) | 12.5 (±3.5) | 22.1 (±9.8) |
| Prick test aeroallergens | n=32 | n=24 | n=6 | n=2 |
| Negative, n (%) | 23 (74.2) | 17 (70.8) | 5 (83.3) | 2 (100) |
| Dust mitesa, n (%) | 7 (22.5) | 6 (25.0) | 1 (16.7) | 0 (0) |
| Planetree, n (%) | 2 (6.5) | 2 (8.3) | 0 (0) | 0 (0) |
| Grasses, n (%) | 1 (3.2) | 1 (4.2) | 0 (0) | 0 (0) |
| Blood workup | n=33 | n=25 | n=6 | n=2 |
| Total IgE, kUI/L (SD) | 62.3 (±93.8) | 63.6 (±96.4) | 74.5 (±102.9) | 11.3 (±4.8) |
| Eosinophils, ×109/L (SD) | 0.27 (±0.22) | 0.27 (±0.22) | 280 (±240.9) | 260 (±282.8) |
| Fibreoptic bronchoscopy | n=9 | n=8 | n=1 | n=0 |
| No impairment, n (%) | 2 (22.2) | 2 (25.0) | 0 (0) | |
| Tracheomalacia, n (%) | 5 (55.6) | 4 (50.0) | 1 (100) | |
| ILO, n (%) | 1 (11.1) | 1 (12.5) | 0 (0) | |
| Lipophages >15% or UAO, n (%) | 2 (22.2) | 2 (25.0) | 0 (0) | |
| Gastroscopy | n=13 | n=10 | n=2 | n=1 |
| No impairment, n (%) | 5 (38.5) | 3 (30.0) | 1 (50) | 1 (100) |
| Gastritis, n (%) | 5 (38.5) | 4 (40.0) | 1 (50) | 0 (0) |
| Esophagitis, n (%) | 1 (7.7) | 1 (10.0) | 0 (0) | 0 (0) |
| Gastric fold atrophy, n (%) | 1 (7.7) | 1 (10.0) | 0 (0) | 0 (0) |
| Hiatal hernia, n (%) | 1 (7.7) | 1 (10.0) | 0 (0) | 0 (0) |
| High-resolution esophageal manometry | ||||
| Motility alterations | n=20 | n=15 | n=3 | n=2 |
| No impairment, n (%) | 5 (25.0) | 3 (20.0) | 2 (66.7) | 0 (0) |
| Hypercontractility tendency, n (%) | 2 (10.0) | 2 (13.3) | 0 (0) | 0 (0) |
| Tertiary waves, n (%) | 1 (5.0) | 0 (0.0) | 1 (33.3) | 0 (0) |
| ≥50% ineffective waves, n (%) | 4 (20.0) | 3 (20.0) | 0 (0) | 1 (50) |
| Ineffective motility, n (%) | 8 (40.0) | 7 (46.7) | 0 (0) | 1 (50) |
| Sphincter/GEJ alterations | ||||
| No impairment, n (%) | 17 (85.0) | 12 (80.0) | 3 (100) | 2 (100) |
| Atypical LES dysfunction, n (%) | 1 (5.0) | 1 (6.7) | 0 (0) | 0 (0) |
| GEJ obstruction, n (%) | 2 (10.0) | 2 (13.3) | 0 (0) | 0 (0) |
| Hiatal hernia, n (%) | 2 (10.0) | 2 (13.3) | 0 (0) | 0 (0) |
| Esophageal 24-h pH | n=20 | n=15 | n=3 | n=2 |
| Pathological GER, n (%) | 9 (45.0) | 7 (46.7) | 1 (33.3) | 1 (50.0) |
Abbreviations: AHT: arterial hypertension; CT, computed tomography; FENO: exhaled fraction of nitric oxide; FEV1: forced expiratory volume in the first second; FVC: forced vital capacity; GEJ: gastroesophageal junction; GER: gastroesophageal reflux; ILO: inducible laryngeal obstruction; IgE: immunoglobulin E; kIU/L: kilo international units per litre; LES: lower esophageal sphincter; ppb: parts per billion; SD, standard deviation; UAO: upper airway obstruction.
The most frequent causes of CC were GER (36.4%; n=12), asthma (15.2%; n=5), and tracheomalacia (12.1%; n=4). While CC resolved after study and with appropriate treatment in 15.2% (n=5) of the included patients, it persisted in the remaining 84.8% (n=28) patients, 72.7% (n=24) and 12.1% (n=4) with RCC and UCC, respectively. Prevalence of biallelic (AAGGG)exp in RFC1 was 7.1% (n=2) in the RCC subgroup, 0% (n=0) in the UCC subgroup, and 33.3% (n=2) in the subgroup with altered Rydel-Seiffer fork results (see Table 2). Note that the 2 cases of early-stage CANVAS had RCC secondary to GER.
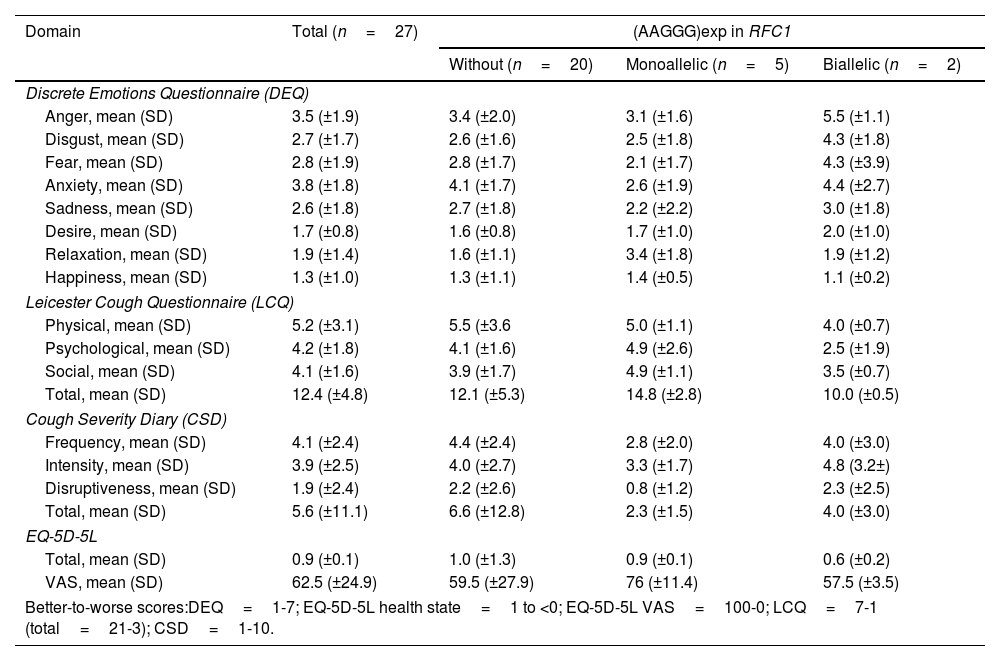
QoL studyQoL questionnaire were administered to 27 patients. Mean DEQ scores signalled anxiety 3.8 (±1.8) and anger 3.5 (±1.9), followed by fear 2.8 (±1.9), disgust 2.7 (±1.7), and sadness 2.6 (±1.8). The mean LCQ overall score was 12.4 (±4.8), with the social 4.1 (±1.6) and psychological 4.2 (±1.8) dimensions showing the highest scores. The mean overall CSD score was 5.6 (±11.1), with frequency having the greatest impact, at 4.1 (±2.4). Finally, the mean EQ-5D-5L part 1 score was 0.9 (±0.1), and the mean part 2 (VAS) score was 62.5 (±24.9). Patients with biallelic (AAGGG)exp scored worst in all 4 QoL studies, while heterozygous (AAGGG)exp carriers had similar score to the patients without that mutation in RFC1 (Table 4).
Chronic cough impact on quality of life: questionnaire results.
| Domain | Total (n=27) | (AAGGG)exp in RFC1 | ||
|---|---|---|---|---|
| Without (n=20) | Monoallelic (n=5) | Biallelic (n=2) | ||
| Discrete Emotions Questionnaire (DEQ) | ||||
| Anger, mean (SD) | 3.5 (±1.9) | 3.4 (±2.0) | 3.1 (±1.6) | 5.5 (±1.1) |
| Disgust, mean (SD) | 2.7 (±1.7) | 2.6 (±1.6) | 2.5 (±1.8) | 4.3 (±1.8) |
| Fear, mean (SD) | 2.8 (±1.9) | 2.8 (±1.7) | 2.1 (±1.7) | 4.3 (±3.9) |
| Anxiety, mean (SD) | 3.8 (±1.8) | 4.1 (±1.7) | 2.6 (±1.9) | 4.4 (±2.7) |
| Sadness, mean (SD) | 2.6 (±1.8) | 2.7 (±1.8) | 2.2 (±2.2) | 3.0 (±1.8) |
| Desire, mean (SD) | 1.7 (±0.8) | 1.6 (±0.8) | 1.7 (±1.0) | 2.0 (±1.0) |
| Relaxation, mean (SD) | 1.9 (±1.4) | 1.6 (±1.1) | 3.4 (±1.8) | 1.9 (±1.2) |
| Happiness, mean (SD) | 1.3 (±1.0) | 1.3 (±1.1) | 1.4 (±0.5) | 1.1 (±0.2) |
| Leicester Cough Questionnaire (LCQ) | ||||
| Physical, mean (SD) | 5.2 (±3.1) | 5.5 (±3.6 | 5.0 (±1.1) | 4.0 (±0.7) |
| Psychological, mean (SD) | 4.2 (±1.8) | 4.1 (±1.6) | 4.9 (±2.6) | 2.5 (±1.9) |
| Social, mean (SD) | 4.1 (±1.6) | 3.9 (±1.7) | 4.9 (±1.1) | 3.5 (±0.7) |
| Total, mean (SD) | 12.4 (±4.8) | 12.1 (±5.3) | 14.8 (±2.8) | 10.0 (±0.5) |
| Cough Severity Diary (CSD) | ||||
| Frequency, mean (SD) | 4.1 (±2.4) | 4.4 (±2.4) | 2.8 (±2.0) | 4.0 (±3.0) |
| Intensity, mean (SD) | 3.9 (±2.5) | 4.0 (±2.7) | 3.3 (±1.7) | 4.8 (3.2±) |
| Disruptiveness, mean (SD) | 1.9 (±2.4) | 2.2 (±2.6) | 0.8 (±1.2) | 2.3 (±2.5) |
| Total, mean (SD) | 5.6 (±11.1) | 6.6 (±12.8) | 2.3 (±1.5) | 4.0 (±3.0) |
| EQ-5D-5L | ||||
| Total, mean (SD) | 0.9 (±0.1) | 1.0 (±1.3) | 0.9 (±0.1) | 0.6 (±0.2) |
| VAS, mean (SD) | 62.5 (±24.9) | 59.5 (±27.9) | 76 (±11.4) | 57.5 (±3.5) |
| Better-to-worse scores:DEQ=1-7; EQ-5D-5L health state=1 to <0; EQ-5D-5L VAS=100-0; LCQ=7-1 (total=21-3); CSD=1-10. | ||||
Abbreviations: EQ-5D-5L: European Quality of Life-5 Dimensions-5 Levels; VAS: visual analogue scale.
Ours is one of the first studies that screens for RFC1 mutations in patients undergoing CC studies. The fact that we did not differentiate at study outset between patients with RCC, UCC, or CC resolved after treatment has made it possible to identify which patients should undergo genetic screening, namely, those in the RCC and UCC subgroups. Our study is also the first to demonstrate the usefulness of Rydel-Seiffer fork results for patient selection for genetic screening, suggesting that this test could be used as criterion for onward referral when genetic screening is not available in all specialized CC consultations (and specially for patients with an altered Rydel-Seiffer fort test), whether in the centre itself or through a referral circuit.
Detecting RFC1 mutations as a means of early diagnosis of CANVAS is important to determine a possible underlying cause of CC that have been classified as UCC. As for future treatments for CANVAS, while there is as yet no cure, some of the symptoms can be treated, using for instance, gabapentin. Genetic diagnosis is important to avoid unnecessary examinations in individuals at risk. Identifying the genetic cause in a family's index case makes it possible to identify other family members at risk of developing or transmitting the disease to their offspring. Furthermore, in the case of CANVAS, the high frequency of carriers in the general population (0.7%)10 would indicate that genetic study is especially important in order to provide genetic and reproductive counselling to family members.
We report prevalence rates for biallelic and monoallelic (AAGGG)exp in RFC1 of 6.1% and 18.2%, respectively, higher than estimated for the general population. Our values are largely in agreement with those reported by Guilleminault et al.,25 with the difference that their biallelic expansion rate was lower, and monoallelic expansion rate was higher, than our rates. Interestingly, that same study described only female heterozygous carriers of (AAGGG)exp in RFC1, whereas one of the monoallelic mutation carriers in our study was a man; a possible explanation is that we studied a broader sample of patients, including a subgroup of patients whose CC resolved after treatment, to which this male patient belonged. Regarding candidates for genetic screening, mutation prevalence was greater in patients with RCC, and was also greater in patients with UCC, but only those with monoallelic expansion (50%; n=2). The higher than expected prevalence of heterozygous (AAGGG)exp carriers is somewhat suggestive of a relationship with CC, although a more robust conclusions would require further studies.
Further evaluation of use of the Rydel-Seiffer fork for early detection of altered peripheral vibratory sensitivity seems appropriate, since 2 of the 6 patients with altered Rydel-Seiffer fork results had a positive result for early-stage CANVAS (biallelic (AAGGG)exp in RFC1, without neurological symptoms). The result of this simple and rapid test could serve as an early sign of CANVAS and enable patient selection for genetic screening, or, where a genetic study was not available, for referral to neurology.
Because no statistically significant differences were found in the clinical or functional characteristics of patients with and without (AAGGG)exp in RFC1, we could not define in further detail which specific patients should undergo genetic testing. In the complementary tests, we only detected a statistically significant difference in relation to chest CT results: patients with no alteration more frequently presented with at least one (AAGGG)exp in RFC1 (p=0.03). Although differences in the GER tests were not statistically significant, the 2 patients with biallelic (AAGGG)exp had impaired esophageal motility, suggesting that further study is needed in this regard to understand the mechanisms underlaying CC.
The questionnaires, which confirmed a poorer QoL that especially affected the psychosocial sphere, corroborate the results of previously published studies.23
The main strength of our study is that it was supported by a multidisciplinary team of neurologists, geneticists, gastroenterologists, and pulmonologists. Limitations include the small sample size – due to the high cost associated with complementary testing and especially genetic studies of patients with CC – and the fact that the study was conducted in a single centre. Another limitation was not having access to long-read sequencing to determine if the heterozygous carrier of (AAGGG)exp/(AAAGG)exp had >500 repeats in the (AAAGG)exp allele. Likewise, we were unable to analyze the possible presence of recently described exceptionally pathogenic motifs associated with CANVAS (AGGGC, AAGGC y AGAGG), although no case has been described to date in Spain. Furthermore, patients with CC showing neurological symptoms compatible with CANVAS were not analyzed because this was a reason for exclusion, and a more exhaustive neurological study would possibly have identified other neurological causes of CC.
ConclusionGenetic screening for (AAGGG)exp in RFC1 should be considered in specialized CC consultations, especially of patients with RCC and UCC. Also meriting consideration in these consultations is use of the Rydel-Seiffer fork, as a low-cost, rapid, and simple complementary test that could identify which patients with CC should be referred for neurological and/or genetic studies. Finally, all CC studies it is important to alert to possible symptoms indicative of CANVAS, in the interest of early diagnosis and of benefit for both the patient and the health system.
Authors’ contributionsSubstantial contributions to study conception and design of the study: EP, VP, LGQ, PG, LQ, FB, MR, ASC, ACL. Acquisition, analysis, and interpretation of data: EP. Drafting the article: EP, ACL. Revising the article critically for important intellectual content and final approval of the version to be submitted: all co-authors.
FundingThis research was supported by a grant from the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) [1144, 2021]. Also for this research, EP was supported by the Catalan Society of Pulmonology (SOCAP) [IIBSP-TOS-2020-143, 2022], LGQ and PG by the Instituto de Salud Carlos III and co-funded by EDRF/FEDER, ‘Una manera de hacer Europa’ under grant from CIBERER [ACCI ER21P1AC705, 2020], and ASC by a grant from the Spanish Ministry of Universities [FPU20/06692, 2020].
Conflicts of interestsEP has received, in the last 3 years, conference travel and attendance expenses from AstraZeneca, Sanofi, Gebro Pharma, Chiesi, FAES Farma, and GSK, and for talks at meetings sponsored by AstraZeneca and GSK, and has received funds/grants for research projects from state agencies, non-profit foundations, and GSK.
VP has received, in the last 3 years, honoraria for speaking at sponsored meetings from Astrazeneca, Boehringer-Ingelheim, Chiesi, Gebro, GSK, Luminova-Medwell, and Sanofi, has received assistance with travel expenses from Astrazeneca and Chiesi, and has acted as a consultant for Astrazeneca, Chiesi, GSK, and Menarini.
LGQ has received conference travel and attendance expenses and fees for talks at meetings sponsored by PTC Therapeutics, and has received funds/grants for research projects from state agencies.
PG, LQ, FB, MR and ASC declare no conflicts of interest.
ACL has received, in the last 3 years, fees for talks at meetings sponsored by AstraZeneca, Zambón, Boehringer Ingelheim, Chiesi, SANOFI, GSK, MSD, Novartis, Orion Pharma, and Sanofi, has received travel and attendance expenses for conferences from Gebro, GSK, Novartis, AstraZeneca, and Sanofi, and has received funds/grants for research projects from several state agencies, non-profit foundations, and AstraZeneca and GSK.
The authors would like to thank Ailish M.J. Maher for translating and reviewing the article.