Pulmonary embolism (PE) poses a significant threat to survival, as roughly 1 in 8 patients succumb either before or shortly after diagnosis.1 The annual incidence of PE is approximately 1/1000 people.2 Chronic kidney disease (CKD) may increase thrombosis risk due to abnormal tissue factor activation,3 potentially worsening renal injury via the protease-activated receptors pathway.4 Individuals with PE and comorbid CKD may have heightened susceptibility to death, but the existing evidence provides inconsistent conclusions.5,6 Higher incidence and mortality of PE in plateau areas7,8 may be attributed to a hypoxia-induced hypercoagulable state9 and cold conditions.10 Additionally, the prevalence of CKD positively correlates with altitude, and hypoxia at high altitudes may heighten the risk of CKD progression.11 However, there is no evidence regarding the association between renal function and mortality risk in acute PE patients in the plateau (altitude exceeding 1500m).
Therefore, we aimed to investigate the association between renal function and the mortality of acute PE patients in plateau areas through a cohort study.
We retrospectively included 553 patients aged ≥18 with clinically suspected acute PE at Qinghai Provincial People's Hospital (the altitude exceeds 2200m, and the average altitude of Xining City, where it belongs, exceeds 3100m) in 2012–2021 (Figure S1). Poor renal function was defined as an estimated glomerular filtration rate (eGFR, estimated based on the CKD Epidemiology Collaboration equation12)<60ml/min/1.73m2.13 Following the Kidney Disease Improving Global Outcomes Organization guidelines, we categorized eGFR into ≥90, 60–89, 45–59, and <45.14 Follow-up began on the date of PE diagnosis, and the endpoint was 30-day death or loss of follow-up. The covariates collected relied on electronic medical records, encompassing sociodemographic details, clinical symptoms, comorbidities, physical and laboratory examinations, echocardiography, and clinical treatment.
Continuous variables with were shown as mean±standard deviation or median (P25, P75), and compared using students’ t-test and Wilcoxon test. Classified variables were displayed based on the number and percentage, and tested based on the chi square test and Fisher exact probability test. We estimated the hazard ratio (HR) and its 95% confidence interval (CI) by using Cox proportional hazard model, and we carried trend tests by modeling the median of each grade as continuous variables.
The study was approved by the Qinghai Provincial People's Hospital (Ethical number: 2022-251). Further information is provided in Supplementary Material.
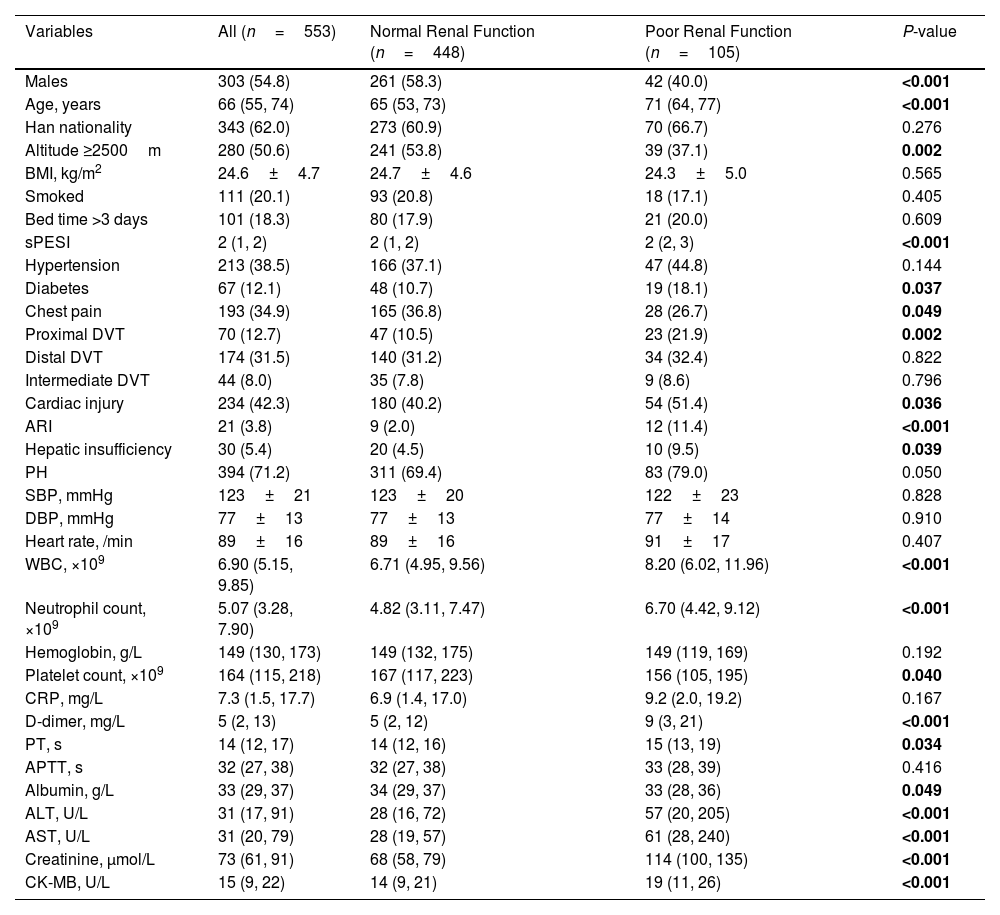
Among 553 participants, the median age was 66 (55, 74) years, with 303 (54.8%) males. Half of the participants resided at an altitude ≥2500m. These patients were characterized by higher age, elevated Simplified Pulmonary Embolism Severity Index (sPESI) scores, increased white blood cell count (WBC), neutrophil count, d-dimer, prothrombin time, alanine transaminase, aspartate aminotransferase (AST), creatinine, and creatine kinase-MB (CK-MB). Additionally, there was a higher proportion of patients with diabetes, an increased likelihood of proximal deep vein thrombosis (DVT), and a higher incidence of cardiac injury, acute renal insufficiency, and hepatic insufficiency. Moreover, those with poor renal function had lower platelets count and albumin, and lower proportion of males, residents living at an altitude ≥2500m, chest pain, and those receiving anticoagulant therapy (Table 1).
Characteristics of Patients With Acute Pulmonary Embolism Group by Renal Function.
| Variables | All (n=553) | Normal Renal Function (n=448) | Poor Renal Function (n=105) | P-value |
|---|---|---|---|---|
| Males | 303 (54.8) | 261 (58.3) | 42 (40.0) | <0.001 |
| Age, years | 66 (55, 74) | 65 (53, 73) | 71 (64, 77) | <0.001 |
| Han nationality | 343 (62.0) | 273 (60.9) | 70 (66.7) | 0.276 |
| Altitude ≥2500m | 280 (50.6) | 241 (53.8) | 39 (37.1) | 0.002 |
| BMI, kg/m2 | 24.6±4.7 | 24.7±4.6 | 24.3±5.0 | 0.565 |
| Smoked | 111 (20.1) | 93 (20.8) | 18 (17.1) | 0.405 |
| Bed time >3 days | 101 (18.3) | 80 (17.9) | 21 (20.0) | 0.609 |
| sPESI | 2 (1, 2) | 2 (1, 2) | 2 (2, 3) | <0.001 |
| Hypertension | 213 (38.5) | 166 (37.1) | 47 (44.8) | 0.144 |
| Diabetes | 67 (12.1) | 48 (10.7) | 19 (18.1) | 0.037 |
| Chest pain | 193 (34.9) | 165 (36.8) | 28 (26.7) | 0.049 |
| Proximal DVT | 70 (12.7) | 47 (10.5) | 23 (21.9) | 0.002 |
| Distal DVT | 174 (31.5) | 140 (31.2) | 34 (32.4) | 0.822 |
| Intermediate DVT | 44 (8.0) | 35 (7.8) | 9 (8.6) | 0.796 |
| Cardiac injury | 234 (42.3) | 180 (40.2) | 54 (51.4) | 0.036 |
| ARI | 21 (3.8) | 9 (2.0) | 12 (11.4) | <0.001 |
| Hepatic insufficiency | 30 (5.4) | 20 (4.5) | 10 (9.5) | 0.039 |
| PH | 394 (71.2) | 311 (69.4) | 83 (79.0) | 0.050 |
| SBP, mmHg | 123±21 | 123±20 | 122±23 | 0.828 |
| DBP, mmHg | 77±13 | 77±13 | 77±14 | 0.910 |
| Heart rate, /min | 89±16 | 89±16 | 91±17 | 0.407 |
| WBC, ×109 | 6.90 (5.15, 9.85) | 6.71 (4.95, 9.56) | 8.20 (6.02, 11.96) | <0.001 |
| Neutrophil count, ×109 | 5.07 (3.28, 7.90) | 4.82 (3.11, 7.47) | 6.70 (4.42, 9.12) | <0.001 |
| Hemoglobin, g/L | 149 (130, 173) | 149 (132, 175) | 149 (119, 169) | 0.192 |
| Platelet count, ×109 | 164 (115, 218) | 167 (117, 223) | 156 (105, 195) | 0.040 |
| CRP, mg/L | 7.3 (1.5, 17.7) | 6.9 (1.4, 17.0) | 9.2 (2.0, 19.2) | 0.167 |
| D-dimer, mg/L | 5 (2, 13) | 5 (2, 12) | 9 (3, 21) | <0.001 |
| PT, s | 14 (12, 17) | 14 (12, 16) | 15 (13, 19) | 0.034 |
| APTT, s | 32 (27, 38) | 32 (27, 38) | 33 (28, 39) | 0.416 |
| Albumin, g/L | 33 (29, 37) | 34 (29, 37) | 33 (28, 36) | 0.049 |
| ALT, U/L | 31 (17, 91) | 28 (16, 72) | 57 (20, 205) | <0.001 |
| AST, U/L | 31 (20, 79) | 28 (19, 57) | 61 (28, 240) | <0.001 |
| Creatinine, μmol/L | 73 (61, 91) | 68 (58, 79) | 114 (100, 135) | <0.001 |
| CK-MB, U/L | 15 (9, 22) | 14 (9, 21) | 19 (11, 26) | <0.001 |
Abbreviations: BMI: body mass index; sPESI: simple pulmonary embolism severity index; DVT: deep venous thrombosis; ARI: acute renal insufficiency; PH: pulmonary hypertension; SBP: systolic blood pressure; DBP: diastolic blood pressure; WBC: white blood cell count; CRP: c-reactive protein; PT: prothrombin time; APTT: activated partial thromboplastin time; ALT: alanine transaminase; AST: aspartate aminotransferase; CK-MB: creatine kinase-MB.
Bold indicates significant differences between groups (P<0.05).
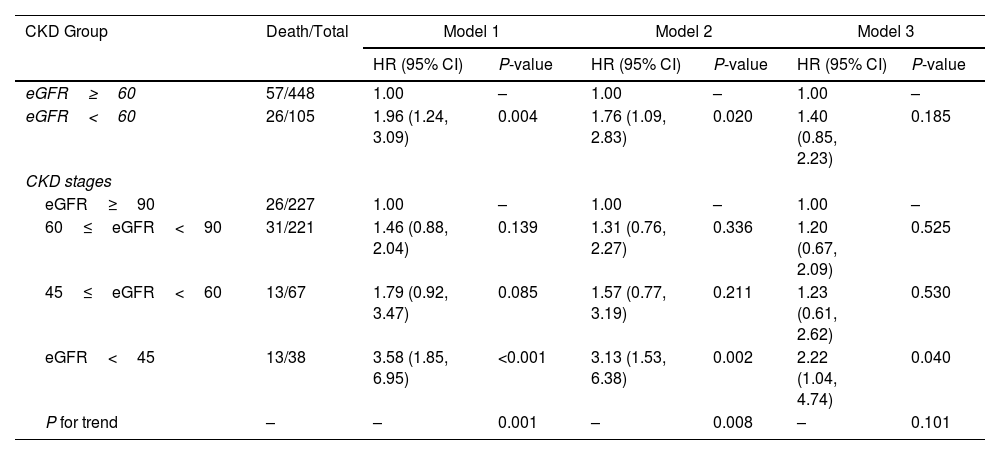
Among patients, the 30-day mortality was 15.01% (95% CI: 12.14%–18.26%). The 30-day mortality rates for patients with normal and poor renal function were 16.8% and 31.3%. The Kaplan–Meier curve (Figure S2) showed poorer survival outcomes for patients with eGFR<60mL/min/1.73m2 than those with eGFR≥60mL/min/1.73m2 (P<0.001). Further, we found that the risk of mortality in patients with eGFR<60mL/min/1.73m2 was 1.96 times (HR=1.96, 95% CI: 1.24–3.09) in crude model, and this association remained significant after adjusting for age and gender. However, this association was not observed after further adjustment for sPESI score, WBC, AST, CK-MB, cardiac injury and chest pain (HR=1.40, 95% CI: 0.85–2.23). Additionally, eGFR<45mL/min/1.73m2 showed a higher risk of death even adjusted for age, gender, sPESI score, WBC, AST, CK-MB, cardiac injury and chest pain (HR=2.22, 95% CI: 1.04–4.74) compared with eGFR≥90mL/min/1.73m2 (Table 2). Stratified analysis showed that patients with cardiac injury (HR=1.95, 95% CI: 1.01–3.76) and hepatic insufficiency (HR=7.60, 95% CI: 1.19–48.58) had stronger association between renal function and mortality. And it was observed that anticoagulant therapy and proximal DVT may modify the association between renal function and mortality, although there is no statistical significance in the subgroups (Table S2).
Association Between Renal Function and Mortality in Patients With Acute Pulmonary Embolism.
| CKD Group | Death/Total | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | ||
| eGFR≥60 | 57/448 | 1.00 | – | 1.00 | – | 1.00 | – |
| eGFR<60 | 26/105 | 1.96 (1.24, 3.09) | 0.004 | 1.76 (1.09, 2.83) | 0.020 | 1.40 (0.85, 2.23) | 0.185 |
| CKD stages | |||||||
| eGFR≥90 | 26/227 | 1.00 | – | 1.00 | – | 1.00 | – |
| 60≤eGFR<90 | 31/221 | 1.46 (0.88, 2.04) | 0.139 | 1.31 (0.76, 2.27) | 0.336 | 1.20 (0.67, 2.09) | 0.525 |
| 45≤eGFR<60 | 13/67 | 1.79 (0.92, 3.47) | 0.085 | 1.57 (0.77, 3.19) | 0.211 | 1.23 (0.61, 2.62) | 0.530 |
| eGFR<45 | 13/38 | 3.58 (1.85, 6.95) | <0.001 | 3.13 (1.53, 6.38) | 0.002 | 2.22 (1.04, 4.74) | 0.040 |
| P for trend | – | – | 0.001 | – | 0.008 | – | 0.101 |
Model 1 was a crude model.
Model 2 further adjusted for age and gender.
Model 3 further adjusted for sPESI (0, ≥1) white blood cell count (<10×109, ≥10×109), AST (<40U/L, ≥40U/L), CK-MB (<0.6 or ≥5.0U/L, 0.6≤CK-MB<5.0U/L), cardiac injury, chest pain.
Our study found that in high-altitude settings, 19.0% of acute pulmonary embolism (PE) patients had concomitant eGFR<60mL/min/1.73m2. Poor renal function may be associated with an increased risk of mortality in PE patients. Additionally, PE patients with cardiac injury and hepatic insufficiency had stronger association between renal function and mortality.
The 30-day mortality rate of PE in our study population was 15.0%, higher than other study reported.1 Multiple factors may explain this finding. Firstly, PE is associated with compromised endothelium and alveolar epithelium due to inflammation from hypoxia, involving pathways and mediators such as hypoxia-inducible factor, vascular endothelial growth factor, endothelin-1, inducible nitric oxide synthase, and regulators like sodium channels, Na-K-ATPase, and aquaporin.15,16 Furthermore, the combination of hypoxia and hypothermia in high-altitude areas can induce hypercoagulability, thereby increasing the risk of thromboembolism.17 Finally, majority of patients in this study had sPESI score of ≥1. Prolonged exposure to high altitude, leading to physiological changes like hyperventilation, increased heart rate, and elevated red blood cell mass,18 is significantly correlated with higher sPESI score.
We found that poor renal function was the risk factor of mortality among acute PE patients, especially in those with eGFR<45mL/min/1.73m2. The abnormal coagulation state observed in CKD patients may provide an explanation for the aforementioned findings.19 Study conducted in plain areas indicated that CKD did not amplify the risk of all-cause death in PE patients,6 while one study reported a 15% increase in the risk of death in PE patients with CKD.5 In terms of mechanisms, the external environment of high-altitude hypoxia can lead to insufficient oxygen supply to the body. Simultaneously, impaired kidney function in the internal environment can result in inadequate secretion of erythropoietin,20 thereby reducing the number of red blood cells and further exacerbating oxygen deficiency. This is especially critical for patients with acute pulmonary embolism, who already experience a decrease in cardiac output,21 intensifying hypoxemia and worsening renal damage, thereby creating a vicious cycle. Furthermore, we observed that the association between renal function and mortality was more pronounced in individuals with cardiac injury and hepatic insufficiency. The combined effect of poor renal function and damage to other organs likely contributes to a higher risk of death.
Remarkably, anticoagulant therapy may modify the association between renal function and mortality. Specifically, individuals received anticoagulant treatment exhibited a stronger association, although the association in the anticoagulant treatment group did not reach statistical significance. This observation may be attributed to the widespread use of low molecular weight heparin in acute PE treatment. Previous studies have indicated that the use of low molecular weight heparin elevates the risk of major bleeding in patients with severe renal insufficiency (defined as creatinine clearance ≤30mL/min).22 This underscores the importance of caution in employing anticoagulation therapy in acute PE patients with comorbid CKD. Exploring safer anticoagulation therapy options is prudent in this population.
The main limitation of this study is the possibility of selection bias. Due to the high medical standards of the hospital conducting this study, the mortality of patients may be overestimated. Secondly, severely PE patients may be unable to undergo CTPA examination, leading to their exclusion from the study, which may underestimate the association between CKD and mortality.
In conclusion, the risk of death within 30 days is higher among PE patients with eGFR<45mL/min/1.73m2. These results suggest PE patients with poor renal function comorbidities who might require more aggressive management.
Ethics approval and consent to participateThe retrospective study involving human participants was approved by the Research Ethics Board at Qinghai Provincial People's Hospital, Qinghai University (Ethical number: 2022-251) and was in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was exempted by the ethics committee of the Qinghai Provincial People's Hospital, Qinghai University.
Authors’ contributionsConception and design: XKF, YJT; Administrative support: XKF, YJT; Provision of study materials or patients: NW, JH; Collection and assembly of data: NW, JH; Data analysis: CLY; Manuscript writing: CLY, YML; Final approval of manuscript: All authors. CLY and YML contributed equally to this article and share first authorship. XKF and YJT contributed equally to this article and shared corresponding authorship.
FundingThis study is supported by The Planning Project of Qinghai Department of Science and Technology (Grant no. 2023-ZJ-719 to Dr Xiaokai Feng); 2023 Kunlun Talents of Qinghai Province High-end Innovation and Entrepreneurship Talent Project-Cultivate leading talents (No. 2023 to Dr XiaoKai Feng).
Conflict of InterestsThe authors declare that they have no competing interests.
We would like to thank the following doctors for taking part in the diagnosis and treatment of patients with PE living at high altitudes from Qinghai Provincial People's Hospital, Qinghai Province, People's Republic China.