Low plasma level of alpha1-antitrypsin (AAT) is an established risk factor for early-onset chronic obstructive lung disease (COPD). However, less attention is given to the levels of AAT in the general population.
MethodsThis is a part of a multicentre, population-based study conducted at 11 sites throughout Spain. Plasma levels of AAT were available for 837 persons with a mean (SD) age of 58.05 (11.3) years: 328-smokers, 272-ex-smokers and 237 non-smokers. Out of 837, 303 (36.2%) had a diagnosis of COPD, 222 (26.5%) had respiratory symptoms but no COPD, and 312 (37.3%) were healthy controls.
ResultsIn the whole cohort, the mean level of plasma AAT was 1.51 (0.47)g/L. Levels were higher in COPD patients [1.55 (0.45)g/L] and individuals with respiratory symptoms [1.57 (0.47)g/L] than in controls [1.43 (0.47)g/L], p<0.001, a finding which persisted after correction for age and CRP. Plasma AAT levels were negatively associated with FEV1/FVC ratio, after adjustment for age, sex, smoking status, CRP, TNFα, fibrinogen and albumin. The risk for COPD was significantly associated with higher AAT levels in univariate and multivariate models, with odds ratios of 1.8 and 1.5, respectively. In the univariate and multivariate models smoking status, gender, and CRP levels were also associated with COPD probability, demonstrating that they act independently.
ConclusionIncreased circulating levels of AAT, similarly to CRP and other markers of systemic inflammation, is an important feature of COPD. Our results highlight a complex interrelationship between levels of AAT and health of respiratory system.
Los niveles plasmáticos bajos de alfa-1 antitripsina (AAT) constituyen un factor de riesgo para el desarrollo temprano de la enfermedad pulmonar obstructiva crónica (EPOC). Sin embargo, se ha prestado una menor atención a los niveles de AAT en la población general.
MétodosEste trabajo forma parte de un estudio poblacional multicéntrico llevado a cabo en 11 centros españoles. Se incluyeron niveles de AAT de 837 personas con una edad media (DE) de 58,05 (11,3) años: 328 fumadores, 272 exfumadores y 237 no fumadores. De los 837, a 303 (36,2%) se les diagnosticó EPOC, 222 (36,5%) presentaron síntomas respiratorios pero no EPOC y 312 (37,3%) eran controles sanos.
ResultadosEn la cohorte total, los niveles plasmáticos medios de AAT fueron 1,51 (0,47)g/l. Los pacientes con EPOC y los individuos con síntomas respiratorios presentaron niveles más elevados (1.55 [0.45]g/l y 1.57 [0.47]g/l respectivamente) que los sujetos control (1.43 [0.47]g/l, p<0.001). Este resultado se mantuvo tras la corrección por edad y niveles de proteína C reactiva (PCR). Los niveles plasmáticos de AAT se asociaron negativamente con la relación FEV1/FVC tras el ajuste por edad, sexo, hábito tabáquico, niveles de PCR, TNFα, fibrinógeno y albúmina. El riesgo de EPOC se asoció significativamente con niveles más elevados de AAT tanto en el modelo univariante como el multivariante, con odds ratios de 1,8 y 1.5 respectivamente. En los modelos univariantes y multivariantes, el hábito tabáquico, el sexo, y los niveles de PCR también se asociaron con la probabilidad de sufrir EPOC, lo cual demostró que se trataba de variables independientes.
ConclusiónUn aumento de los niveles circulantes de AAT, al igual que los niveles de PCR y otros marcadores de inflamación sistémica, son características importantes de la EPOC. Nuestros resultados evidencian la compleja interrelación existente entre los niveles de AAT y la salud del aparato respiratorio.
Alpha1-antitrypsin (AAT) is an acute phase protein, major inhibitor of neutrophil serine proteases like elastase but also considered as a regulatory protein of broad immune and inflammatory responses. Experimental data provide evidence that AAT assists host defences by neutralizing inflammation-induced molecules (such as proteases, cytokines and oxidants), by diminishing the pro-inflammatory responses of circulating leukocytes, and by forestalling endothelial activation.1,2
The clinical importance of AAT is highlighted in individuals with inherited AAT deficiency, who exhibit high risk for developing early-onset pulmonary emphysema and liver disease at any age.3 Because inherited AAT deficiency is a well-established genetic risk factor for chronic obstructive pulmonary disease (COPD), most of the clinical studies focus on reduced plasma levels and biological activity of AAT protein in cohorts of COPD. Hitherto, less attention given to the levels of plasma AAT in the general population and in COPD patients without inherited AAT deficiency.
Here we used data from the EPI-SCAN, a multicentre, epidemiological study conducted in Spain.4 Our aim was to determine plasma AAT levels in a cohort of participants diagnosed with COPD, having respiratory symptoms-presence of chronic cough and sputum production but no clinical diagnosis of COPD, and the reference group, those with normal spirometry and without respiratory symptoms, considered healthy persons. We also wanted to evaluate independent predictors of AAT levels including age, lung function measures, smoking, and clinical laboratory data.
MethodsDesign of the studyThe present study is a part of the EPI-SCAN study, which was a multicentre, cross-sectional, population-based, observational study conducted at 11 sites in 10 Spanish cities (Barcelona, Burgos, Córdoba, Huesca, Madrid (two areas), Requena, Sevilla, Oviedo, Vic and Vigo) representing different geographic, climatic and socio-economic regions. The main objective of the study was to investigate the prevalence of COPD in Spain in individuals between 40 and 80 years of age. The protocol of the EPI-SCAN study has been published elsewhere.4 Fieldwork and all methods have been described previously.5 The corresponding ethics committees approved the study and all participants gave written informed consent.
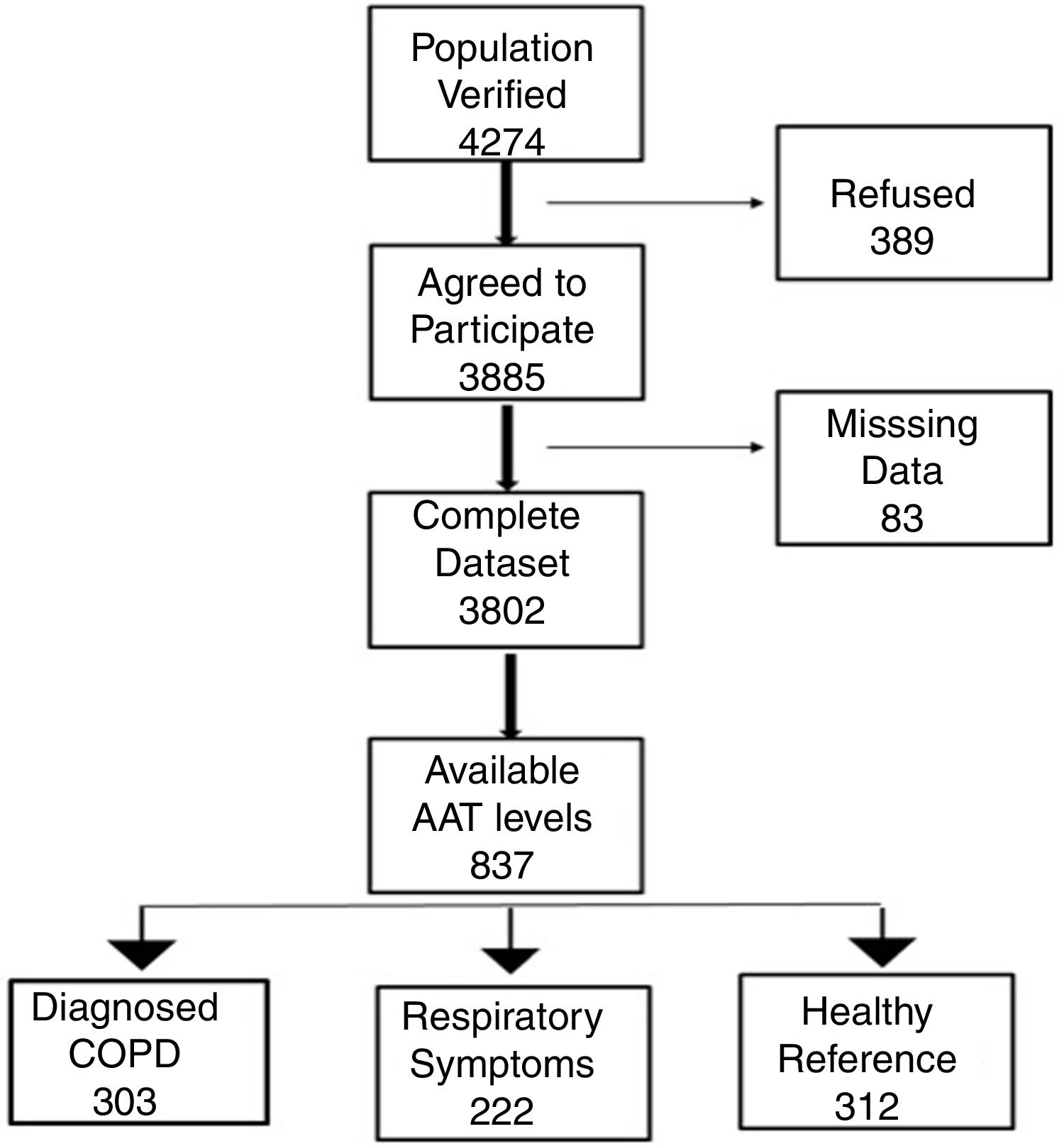
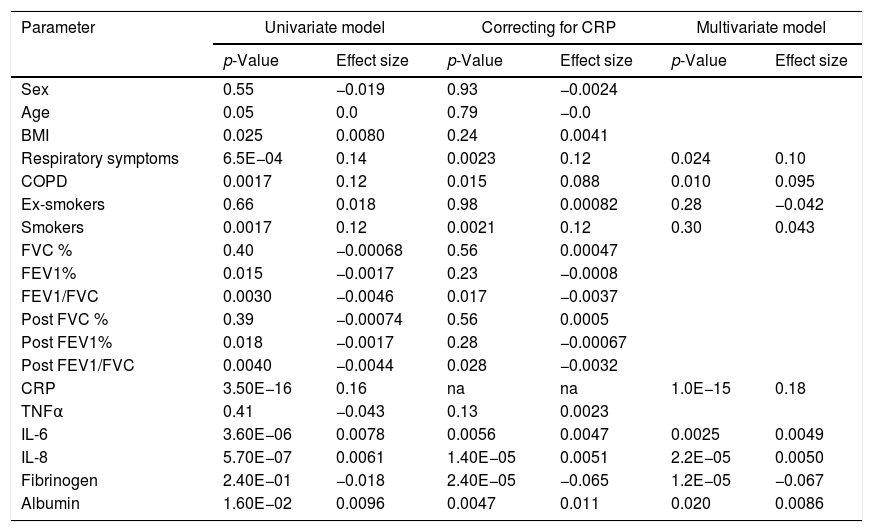
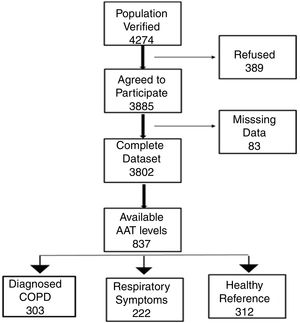
Study participantsThe final recruited population consisted of 3802 non-institutionalized participants from 40 to 80 years of age. The definition of COPD was a post-bronchodilator FEV1/FVC ratio <0.70. Subjects considered not to have COPD had a post-bronchodilator FEV1/FVC ratio ≥0.70.6 The blood sample we obtained from all participants classified as having COPD. To avoid excessive testing of the non-COPD population, each study center collected equal number of blood samples from non-COPD subjects recruited consecutively in order to avoid selection bias. Exclusion criteria included a previous diagnosis of acute myocardial infarction, angina, congestive heart failure, cancer, hepatic cirrhosis, chronic renal failure, rheumatoid arthritis or any other systemic inflammatory disease. In addition, we identified a control group of non-COPD subjects after applying the same comorbidity exclusion criteria and specific exclusion criteria of any respiratory symptoms as per the European Coal and Steel Community (ECSC) questionnaire and regularly prescribed medications.7Fig. 1 illustrates our cohort.
ProceduresAt each study center, lung function tests were performed using the same equipment according to the current recommendations.8 Spirometry was performed before and 15–30min after salbutamol inhalation. Baseline dyspnea was assessed by the Modified Medical Research Council (MMRC) scale,9 and subjects completed the ECSC questionnaire of respiratory symptoms.10
Blood samples were collected using standardized procedures and stored at −80°C before sending for the centralized analysis to a single laboratory (Hospital Clinic, Barcelona, Spain). Plasma levels of TNFα, IL-6 and IL-8 were determined in duplicate with a high sensitivity enzyme-linked immunosorbent assays (Biosource, Nivelles, Belgium). We assessed plasma levels of CRP by the latex-enhanced immunonephelometry (Siemens, Dublin, Ireland), AAT by a particle-enhanced immunonephelometry (Siemens, Marburg, Germany), albumin by the bromocresol green method (Siemens, Dublin, Ireland) and fibrinogen by using a coagulation analyzer (Roche, Manheim, Germany). Specific details about the analytic procedures described elsewhere.7
Statistical analysisData analysis performed using R, a free software environment for statistical computing and graphics. Statistical significance between subject categories with regard to demographic characteristics and laboratory values was analyzed with ANOVA. Specific pairwise comparisons of subject categories were assessed with the t-test. Least squares linear regression was applied to assess the associations of the dependent variables, such as AAT levels and lung function (post-bronchodilator spirometry), with various other clinical parameters as independent variables. Modeling COPD status as a function of AAT levels and other clinical parameters was performed using logistic regression.
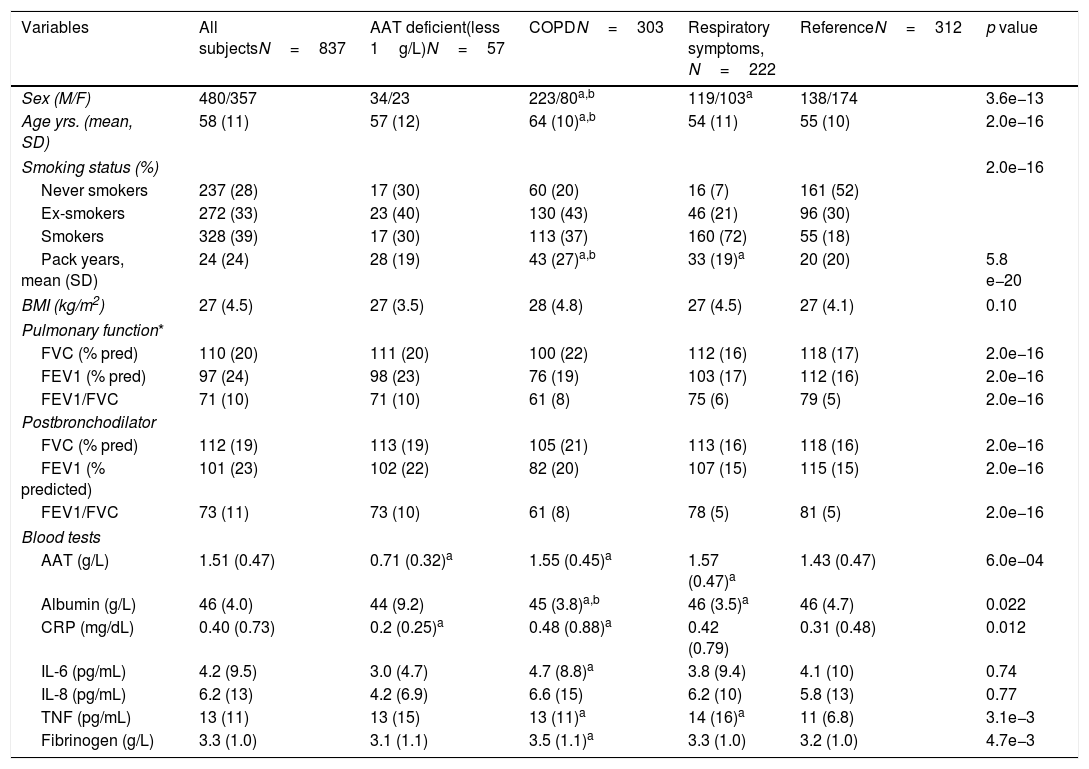
ResultsCharacteristics of study participantsOut of 3802 participants, 837 (22%) had available plasma levels of AAT. Of them, 303 (36.2%) had COPD diagnosis, 222 (26.5%) had respiratory symptoms but not COPD, and 312 (37.3%) were healthy, with normal spirometry and without respiratory symptoms, considered as a reference group. The mean (SD) age of cohort was 58.0 (11.3) years. The main characteristics of the participants described in Table 1.
General characteristics of the whole study group and subgroups.
| Variables | All subjectsN=837 | AAT deficient(less 1g/L)N=57 | COPDN=303 | Respiratory symptoms, N=222 | ReferenceN=312 | p value |
|---|---|---|---|---|---|---|
| Sex (M/F) | 480/357 | 34/23 | 223/80a,b | 119/103a | 138/174 | 3.6e−13 |
| Age yrs. (mean, SD) | 58 (11) | 57 (12) | 64 (10)a,b | 54 (11) | 55 (10) | 2.0e−16 |
| Smoking status (%) | 2.0e−16 | |||||
| Never smokers | 237 (28) | 17 (30) | 60 (20) | 16 (7) | 161 (52) | |
| Ex-smokers | 272 (33) | 23 (40) | 130 (43) | 46 (21) | 96 (30) | |
| Smokers | 328 (39) | 17 (30) | 113 (37) | 160 (72) | 55 (18) | |
| Pack years, mean (SD) | 24 (24) | 28 (19) | 43 (27)a,b | 33 (19)a | 20 (20) | 5.8 e−20 |
| BMI (kg/m2) | 27 (4.5) | 27 (3.5) | 28 (4.8) | 27 (4.5) | 27 (4.1) | 0.10 |
| Pulmonary function* | ||||||
| FVC (% pred) | 110 (20) | 111 (20) | 100 (22) | 112 (16) | 118 (17) | 2.0e−16 |
| FEV1 (% pred) | 97 (24) | 98 (23) | 76 (19) | 103 (17) | 112 (16) | 2.0e−16 |
| FEV1/FVC | 71 (10) | 71 (10) | 61 (8) | 75 (6) | 79 (5) | 2.0e−16 |
| Postbronchodilator | ||||||
| FVC (% pred) | 112 (19) | 113 (19) | 105 (21) | 113 (16) | 118 (16) | 2.0e−16 |
| FEV1 (% predicted) | 101 (23) | 102 (22) | 82 (20) | 107 (15) | 115 (15) | 2.0e−16 |
| FEV1/FVC | 73 (11) | 73 (10) | 61 (8) | 78 (5) | 81 (5) | 2.0e−16 |
| Blood tests | ||||||
| AAT (g/L) | 1.51 (0.47) | 0.71 (0.32)a | 1.55 (0.45)a | 1.57 (0.47)a | 1.43 (0.47) | 6.0e−04 |
| Albumin (g/L) | 46 (4.0) | 44 (9.2) | 45 (3.8)a,b | 46 (3.5)a | 46 (4.7) | 0.022 |
| CRP (mg/dL) | 0.40 (0.73) | 0.2 (0.25)a | 0.48 (0.88)a | 0.42 (0.79) | 0.31 (0.48) | 0.012 |
| IL-6 (pg/mL) | 4.2 (9.5) | 3.0 (4.7) | 4.7 (8.8)a | 3.8 (9.4) | 4.1 (10) | 0.74 |
| IL-8 (pg/mL) | 6.2 (13) | 4.2 (6.9) | 6.6 (15) | 6.2 (10) | 5.8 (13) | 0.77 |
| TNF (pg/mL) | 13 (11) | 13 (15) | 13 (11)a | 14 (16)a | 11 (6.8) | 3.1e−3 |
| Fibrinogen (g/L) | 3.3 (1.0) | 3.1 (1.1) | 3.5 (1.1)a | 3.3 (1.0) | 3.2 (1.0) | 4.7e−3 |
a: vs reference; b: vs respiratory symptoms.
There were more men within the COPD group than in those with respiratory symptoms or healthy (73.6% vs 53.6% and 44.2%, respectively). Moreover, COPD group was older and had more smokers and ex-smokers relative to other groups. BMI was not significantly different among the groups. As expected, lung function was significantly impaired in COPD individuals compared to the healthy reference group, and there was a wide range of COPD severity in our cohort. Although to a lesser degree, lung function was also decreased in individuals with respiratory symptoms compared to the reference group.
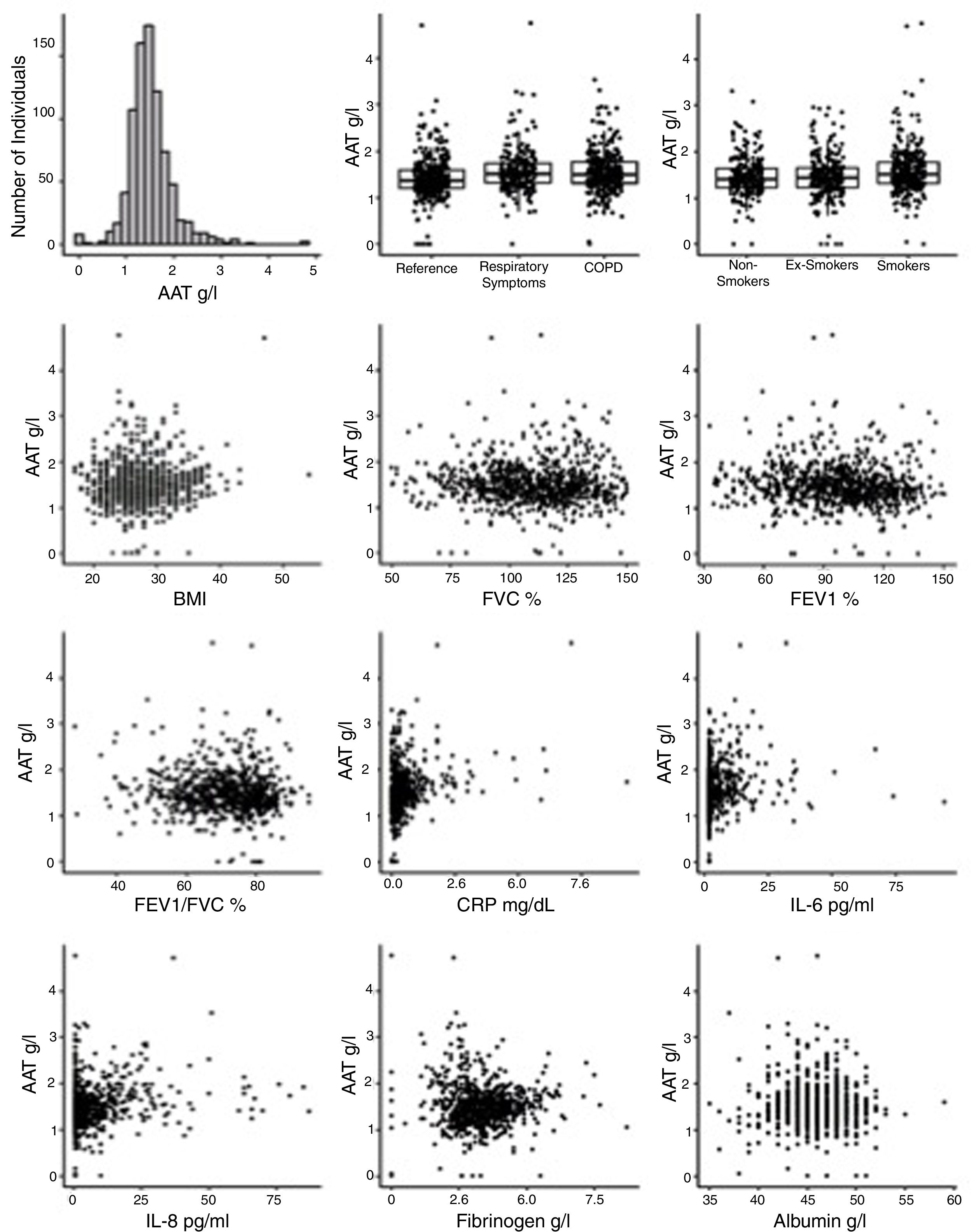
Plasma levels of AAT and other markers of systemic inflammationIn the entire cohort, levels of AAT were 1.51 (0.47)g/L, with no significant differences between men and women. Notably, a subset of cases (57 or 6.8%) exhibited particularly low levels of plasma AAT (below 1g/L): 20 within COPD, 9 within those having respiratory symptoms and 28 within the reference group. Unfortunately, in this cross-sectional study, genotypes of AAT were not determined and therefore the correlation between genotype and low AAT protein levels could not be analyzed. Removing low-AAT cases as outliers did not affect the observations, and these 57 cases were included in the final analysis. When compared to reference group, subjects with respiratory symptoms or COPD showed higher plasma levels of AAT (p<0.001). As illustrated in Table 1, analysis of other plasma proteins revealed that COPD subjects had significantly higher plasma levels of CRP, IL-6 and fibrinogen but lower albumin concentrations if compared to the reference group. Plasma levels of TNFα were higher in the COPD and respiratory symptoms groups compared to reference, whereas IL-8 did not significantly differ among the groups.
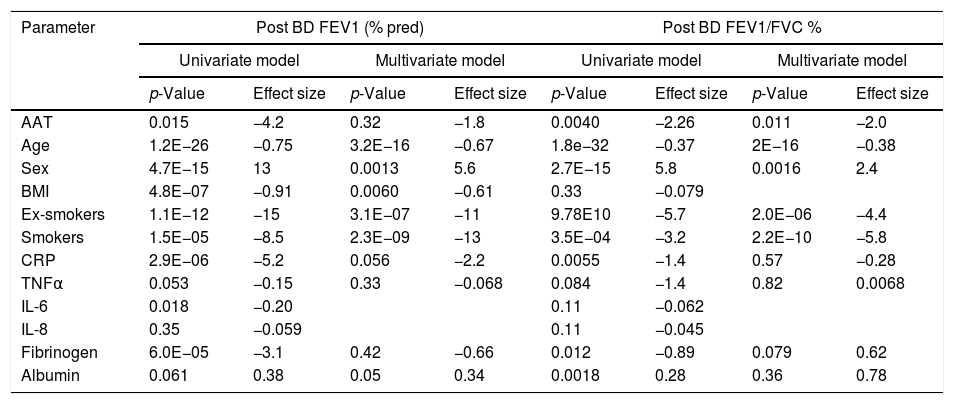
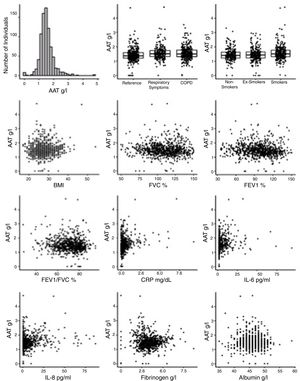
Plasma AAT levels in relationship to respiratory health and laboratory measuresWe next examined the relationship between plasma AAT levels and clinical/laboratory measures. For this, we applied three types of linear regression models: univariate, correcting for CRP, and multivariate. As shown in Table 2 and Fig. 2, plasma AAT levels were significantly affected by cohort (healthy, respiratory symptoms or COPD), smoking status (smokers but not ex-smokers), lung function tests, and levels of CRP, IL-6, IL-8, fibrinogen and albumin, but not by TNFα.
Associations of plasma AAT levels with clinical parameters.
| Parameter | Univariate model | Correcting for CRP | Multivariate model | |||
|---|---|---|---|---|---|---|
| p-Value | Effect size | p-Value | Effect size | p-Value | Effect size | |
| Sex | 0.55 | −0.019 | 0.93 | −0.0024 | ||
| Age | 0.05 | 0.0 | 0.79 | −0.0 | ||
| BMI | 0.025 | 0.0080 | 0.24 | 0.0041 | ||
| Respiratory symptoms | 6.5E−04 | 0.14 | 0.0023 | 0.12 | 0.024 | 0.10 |
| COPD | 0.0017 | 0.12 | 0.015 | 0.088 | 0.010 | 0.095 |
| Ex-smokers | 0.66 | 0.018 | 0.98 | 0.00082 | 0.28 | −0.042 |
| Smokers | 0.0017 | 0.12 | 0.0021 | 0.12 | 0.30 | 0.043 |
| FVC % | 0.40 | −0.00068 | 0.56 | 0.00047 | ||
| FEV1% | 0.015 | −0.0017 | 0.23 | −0.0008 | ||
| FEV1/FVC | 0.0030 | −0.0046 | 0.017 | −0.0037 | ||
| Post FVC % | 0.39 | −0.00074 | 0.56 | 0.0005 | ||
| Post FEV1% | 0.018 | −0.0017 | 0.28 | −0.00067 | ||
| Post FEV1/FVC | 0.0040 | −0.0044 | 0.028 | −0.0032 | ||
| CRP | 3.50E−16 | 0.16 | na | na | 1.0E−15 | 0.18 |
| TNFα | 0.41 | −0.043 | 0.13 | 0.0023 | ||
| IL-6 | 3.60E−06 | 0.0078 | 0.0056 | 0.0047 | 0.0025 | 0.0049 |
| IL-8 | 5.70E−07 | 0.0061 | 1.40E−05 | 0.0051 | 2.2E−05 | 0.0050 |
| Fibrinogen | 2.40E−01 | −0.018 | 2.40E−05 | −0.065 | 1.2E−05 | −0.067 |
| Albumin | 1.60E−02 | 0.0096 | 0.0047 | 0.011 | 0.020 | 0.0086 |
The multivariate relationship between AAT levels and clinical measures (graphical presentation of the data analysis presented in Table 2).
Levels of AAT were higher in individuals with COPD or with respiratory symptoms compared to the reference group, an effect that persisted after age and CRP correction, as well as in the multivariate model. Age did not show an impact on AAT levels. Plasma levels of CRP, IL-6, IL-8 and albumin were positively associated, whereas levels of fibrinogen were negatively associated with AAT. After correcting for CRP levels, most parameters remained significantly associated with AAT levels with the exception of FEV1 (pre and post-bronchodilators). The multivariate analysis included smoking status; post FEV/FVC, CRP, IL-6, IL-8, fibrinogen and albumin. Even when modeled jointly, each of these parameters contributed significantly to the multivariate model. This implies that there is an independent relationship of each of these factors to plasma AAT levels.
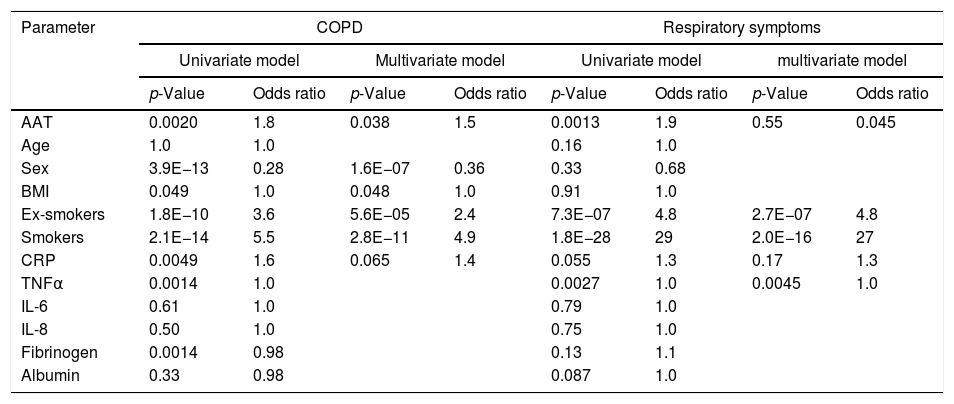
The relationship between AAT levels and measures of airway obstructionWe also examined whether our data above support the relationship between plasma AAT levels and lung function. To do this, we modeled lung function measures, FEV1 and FEV1/FVC, as a function of AAT and other relevant analytics in both univariate and multivariate models (Table 3). In the univariate model, FEV1 was significantly and inversely associated with AAT levels, but the effect was weaker relative to factors like sex, BMI and smoking status. When combining these terms in a multivariate model, the above independent effect of AAT was diminished to non-significant level (p<0.32). However, the association between plasma AAT levels and lung function, as measured by FEV1/FVC ratio, was more robust. Here the inverse association was stronger and remained independently significant even when correcting for other associated factors such as age, sex, smoking status, CRP, TNFα, fibrinogen and albumin. Incidental to the associations of AAT with lung function, we also observe significant decreases in lung function with increased age, smoking, BMI, and CRP. Lung function (FEV1) was positively associated with being female and albumin.
Lung function tests as a function of AAT and other parameters.
| Parameter | Post BD FEV1 (% pred) | Post BD FEV1/FVC % | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate model | Multivariate model | Univariate model | Multivariate model | |||||
| p-Value | Effect size | p-Value | Effect size | p-Value | Effect size | p-Value | Effect size | |
| AAT | 0.015 | −4.2 | 0.32 | −1.8 | 0.0040 | −2.26 | 0.011 | −2.0 |
| Age | 1.2E−26 | −0.75 | 3.2E−16 | −0.67 | 1.8e−32 | −0.37 | 2E−16 | −0.38 |
| Sex | 4.7E−15 | 13 | 0.0013 | 5.6 | 2.7E−15 | 5.8 | 0.0016 | 2.4 |
| BMI | 4.8E−07 | −0.91 | 0.0060 | −0.61 | 0.33 | −0.079 | ||
| Ex-smokers | 1.1E−12 | −15 | 3.1E−07 | −11 | 9.78E10 | −5.7 | 2.0E−06 | −4.4 |
| Smokers | 1.5E−05 | −8.5 | 2.3E−09 | −13 | 3.5E−04 | −3.2 | 2.2E−10 | −5.8 |
| CRP | 2.9E−06 | −5.2 | 0.056 | −2.2 | 0.0055 | −1.4 | 0.57 | −0.28 |
| TNFα | 0.053 | −0.15 | 0.33 | −0.068 | 0.084 | −1.4 | 0.82 | 0.0068 |
| IL-6 | 0.018 | −0.20 | 0.11 | −0.062 | ||||
| IL-8 | 0.35 | −0.059 | 0.11 | −0.045 | ||||
| Fibrinogen | 6.0E−05 | −3.1 | 0.42 | −0.66 | 0.012 | −0.89 | 0.079 | 0.62 |
| Albumin | 0.061 | 0.38 | 0.05 | 0.34 | 0.0018 | 0.28 | 0.36 | 0.78 |
A number of outliers with a high AAT level and high FEV1/FVC (Fig. 2), allows suggesting that responders with high AAT may actually be protected from severe COPD when adjusting for smoking and age. Therefore, only for individuals with high AAT levels, we investigated the associations between AAT and FEV1/FVC. We were unable to observe any indications of protection although the low numbers of cases available leaves us underpowered for any conclusive statements.
Dose response relationship between smoke and AAT levelWe have explored whether there is a dose response relationship between smoke and AAT level. By using AAT levels and smoking status as an interaction term in the model, we observed no significant interactions in this cohort. However, we observed for the cohort as a whole, that pack-years were associated with decreased lung function in our multivariate model (p<3.3 E10−7). When used in an interaction term with AAT, we also observed that this interaction was significant (p<0.06 for FEV1 and 0.009 for FEV/FVC).
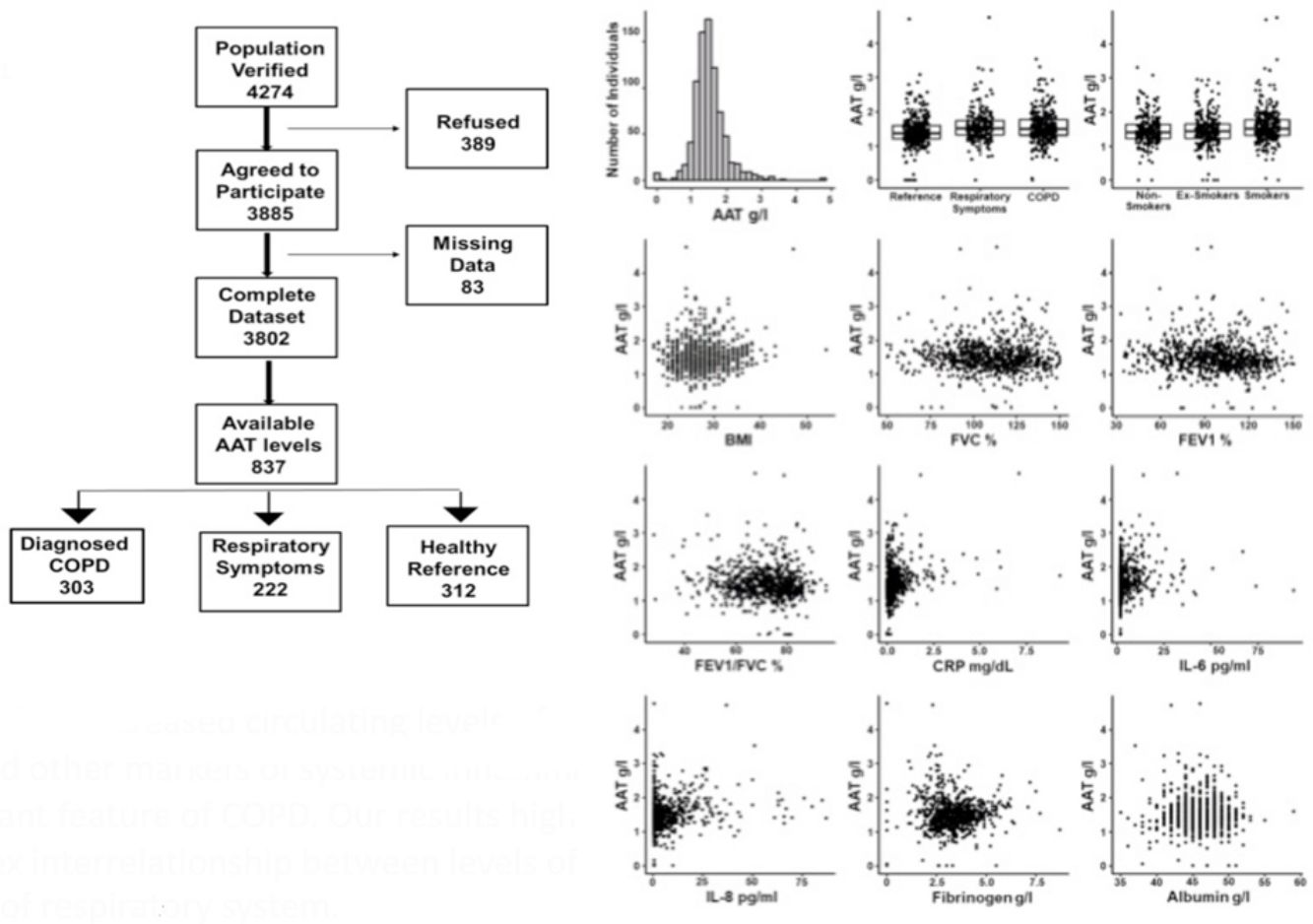
COPD as a function of plasma AAT levels: binomial logistic regression analysisBased on the findings above, we were interested in the association of AAT levels with COPD in the context of the other measured clinical variables. To this end, we modeled COPD diagnosis as a function of plasma AAT levels and other measured parameters using binomial logistic regression (Table 4). The risk for COPD significantly increased with higher AAT levels in both the univariate and multivariate models, with odds ratios of 1.8 and 1.5, respectively. The univariate model demonstrated that sex, smoking status and CRP levels were associated with COPD. In the multivariate model, these parameters continued to be associated with COPD probability, demonstrating that they act independently. Similarly, we looked at the AAT levels as a risk for respiratory symptoms. Here, we did not observe a significant association with AAT after accounting for other factors, such as smoking status, TNFα, IL-8, and IL-6. Herein smoking status appears to be a strongest risk factor for respiratory symptoms in a non-COPD population.
Health status as a function of clinical parameters.
| Parameter | COPD | Respiratory symptoms | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate model | Multivariate model | Univariate model | multivariate model | |||||
| p-Value | Odds ratio | p-Value | Odds ratio | p-Value | Odds ratio | p-Value | Odds ratio | |
| AAT | 0.0020 | 1.8 | 0.038 | 1.5 | 0.0013 | 1.9 | 0.55 | 0.045 |
| Age | 1.0 | 1.0 | 0.16 | 1.0 | ||||
| Sex | 3.9E−13 | 0.28 | 1.6E−07 | 0.36 | 0.33 | 0.68 | ||
| BMI | 0.049 | 1.0 | 0.048 | 1.0 | 0.91 | 1.0 | ||
| Ex-smokers | 1.8E−10 | 3.6 | 5.6E−05 | 2.4 | 7.3E−07 | 4.8 | 2.7E−07 | 4.8 |
| Smokers | 2.1E−14 | 5.5 | 2.8E−11 | 4.9 | 1.8E−28 | 29 | 2.0E−16 | 27 |
| CRP | 0.0049 | 1.6 | 0.065 | 1.4 | 0.055 | 1.3 | 0.17 | 1.3 |
| TNFα | 0.0014 | 1.0 | 0.0027 | 1.0 | 0.0045 | 1.0 | ||
| IL-6 | 0.61 | 1.0 | 0.79 | 1.0 | ||||
| IL-8 | 0.50 | 1.0 | 0.75 | 1.0 | ||||
| Fibrinogen | 0.0014 | 0.98 | 0.13 | 1.1 | ||||
| Albumin | 0.33 | 0.98 | 0.087 | 1.0 | ||||
The present study includes Spanish population-based cohort of 837 participants with measured plasma levels of AAT and their detailed characterization, which allows investigating factors associated with circulating AAT, as well as the association of the AAT levels with health indicators of the respiratory system. Importantly, based on CRP measurements we were able to control for systemic inflammation, as a potential confounder.11
Although determined AAT levels were within the normal reference range [mean (SD) 1.51 (0.47)g/L], participants with stable COPD or with respiratory symptoms showed 8.4% and 9.8% higher levels of plasma AAT than healthy participants [1.43 (0.47)g/L], a finding which was statistically significant and persisted after correction for age and CRP. Results from both the univariate and multivariate models revealed that the risk for COPD was significantly associated with higher circulating levels of AAT. This relationship remained significant even after correction for other factors associated with COPD, such as age, sex, smoking status, CRP, TNFα, fibrinogen and albumin. In fact, we did not observe a significant association between plasma levels of AAT and respiratory symptoms. Herein, smoking status appears to be the strongest risk factor for respiratory symptoms in non-COPD subjects.
Our present findings are in line with those of a Copenhagen General Population study demonstrating that elevated plasma AAT levels were associated with COPD and increased risk of exacerbations.12 Similarly the epidemiologic study published by Thomsen et al.13 found higher plasma levels of AAT in never smokers with COPD than in never smokers without COPD. Furthermore, in small cohorts, others and ourselves previously reported higher serum levels of AAT in COPD patients than in controls, independent of genetic AAT-variant.14,15 Altogether, previous and our current results provide evidence that higher levels of AAT are associated with COPD.
It is important to point out that in the population-based Swiss SAPALDIA cohort, circulating AAT levels were inversely correlated with FEV1, but only in the absence of CRP adjustment.16 Accordingly, in our study FEV1 was also significantly and inversely associated with AAT levels, but only without adjustment for other factors like CRP, sex, BMI, and smoking status. However, the negative association between plasma AAT levels and lung function, as measured by FEV1/FVC ratio, was stronger and remained independently significant after adjusting for other associated factors, such as sex, smoking status, CRP, TNFα, fibrinogen and albumin. The ratio of FEV1/FVC is the major determinant of airflow obstruction and the diagnosis of COPD. Therefore, we think that the association between plasma levels of AAT and lung function may depend on the tests performed and used for data analysis. Further studies are required to confirm our suggestion.
COPD is chronic inflammatory disease. Several studies proposed that elevated circulating inflammatory markers, such as CRP, fibrinogen and inflammatory cytokines (TNFα, IL-6, and IL-8) predict morbidity and mortality of patients with COPD.17,18 Specifically CRP, a clinical indicator of inflammation,19 has repeatedly been recommended as a useful predictor of clinical outcomes in patients with COPD.20–22 Large prospective studies following healthy people for up to 20 years found that a chronically elevated CRP is associated with a progressive loss of lung function and a greater risk of COPD.23,24 A recent study by Bradford et al. found a strong association between IL-6 and COPD status, airflow limitation and emphysema progression.25
By using the univariate and multivariate models, we found that the risk for COPD significantly increased with higher levels of AAT. In the multivariate model, we also found that sex, smoking status and CRP levels were associated with having COPD, suggesting that they act independently. Similar to CRP or other acute phase proteins, the synthesis of AAT regulated through the action of pro-inflammatory cytokines, specifically IL-6.26 Therefore, in parallel to increased IL-6 and CRP levels; the low-grade upregulation of AAT in stable COPD may also be useful as a long-term predictor of future outcomes.
The above-mentioned results emphasize that COPD is associated with low-grade systemic inflammation and that circulating cytokines and acute-phase proteins function adversely by maintaining activation of innate immune hyper-responsiveness in the circulation as well as in the lungs. The systemic inflammation in COPD may also explain, at least in part, extra-pulmonary manifestations.27
Our study has some strengths and limitation. The strengths include the sample size, population-based design, and the detailed characterization of the participants, which allowed us to investigate factors associated with circulating AAT levels as well as the association of the AAT with lung function and COPD. The limitations to be considered are that (i) biomarkers were assessed at only a single time point and thus we could not assess their fluctuation during the course of the disease, (ii) the genotypes of AAT were not determined, and (iii) although significant, the differences in AAT levels between groups were of small magnitude.
We believe that combining information from AAT levels and other acute phase reactants may improve the prediction of risk and/or progression in COPD. It may also help to explain controversies as to whether -systemic inflammation in COPD represents ‘spill over’ from the lung inflammation or is a chronic systemic process.28 According to recently reported mouse model, the liver may amplify innate immune responsiveness in the lungs after bacterial infection. This latter seems to depend on increased release of IL-6 by alveolar macrophages along with other acute-phase proteins.29 The liver is a main organ producing and releasing into the circulation acute phase proteins, including AAT and CRP. Different studies demonstrated that activated lung epithelial cells, alveolar macrophages and granulocytes also contribute to the pool of circulating AAT. Whether higher plasma levels of AAT in patients with COPD mirror ‘spill over’ from the lungs or increased release by the liver, we cannot yet answer. Inflammatory process initiated in the lung or elsewhere, may lead to the injury in the alveolar space.30 In support, results from a Norwegian general population study revealed that increased levels of AAT were associated with a decrease in lung diffusion capacity.31 Further studies would be necessary to confirm our results and elucidate the source of increased plasma AAT in COPD. Finally, the interest in AAT as a prognostic marker may help to increase the determinations of this protein in COPD and eventually reduce the under diagnosis of the AAT deficiency.32
ConclusionThere is a considerable inter-individual variation in the circulating levels of AAT, and there is no doubt that this plays an important role during health and diseases.1 Nevertheless, the variability of plasma AAT levels in population-based studies and the possible incorporation of this variability in disease risk assessment remain largely unexplored. A large body of evidence shows that the degree of risk for COPD inversely relates to the plasma level of AAT according to the AAT genotypes: NullNull>ZZ>SZ>MZ.33,34 Our study confirms that increased circulating levels of AAT, similarly to CRP, IL-6 and other putative markers of systemic inflammation, is an important feature of COPD. Hence, determination of AAT concentrations can be an important diagnostic and/or monitoring test not only for people with suspected inherited AAT deficiency, but also for a general population with and- without COPD.
Conflicts of interestMiriam Barrecheguren has received speaker fees from Grifols, Menarini, and consulting fees from Novartis and Gebro Pharma, unrelated to this manuscript. Marc Miravitlles has received speaker or consulting fees from AstraZeneca, Bial, Boehringer Ingelheim, Chiesi, Cipla, CSL Behring, Laboratorios Esteve, Ferrer, Gebro Pharma, GlaxoSmithKline, Grifols, Menarini, Mereo Biopharma, Novartis, pH Pharma, Rovi, TEVA, Verona Pharma and Zambon, and research grants from GlaxoSmithKline and Grifols, unrelated to this manuscript.
The EPI-SCAN study has been funded by GlaxoSmithKline Spain.
The organization of the EPI-Scan study was as follows:
Scientific Committee: Julio Ancochea. Hospital La Princesa (Madrid), Carlos Badiola and Guadalupe Sánchez, GlaxoSmithkline (Madrid), Enric Duran. Institut Municipal d’Investigació Mèdica (IMIM) (Barcelona), Francisco García Río. Hospital La Paz (Madrid), Marc Miravitlles. Hospital Universitari Vall d’Hebron (Barcelona), Luis Muñoz. Hospital Reina Sofía (Córdoba), Víctor Sobradillo. Hospital de Cruces (Bilbao), Joan B Soriano. Fundació Caubet-CIMERA (Bunyola, Illes Balears). Participating sites and coordinators: Julio Ancochea. Hospital La Princesa (Madrid), Luis Borderias. Hospital San Jorge (Huesca), Francisco García Río. Hospital La Paz (Madrid), Jaime Martínez. Hospital Central de Asturias (Oviedo), Teodoro Montemayor. Hospital Virgen de la Macarena (Sevilla), Luis Muñoz. Hospital Reina Sofía (Córdoba), Luis Piñeiro. Hospital Xeral Cies (Vigo), Joan Serra. Hospital General de Vic (Vic, Barcelona), Juan José Soler-Cataluña. Hospital General de Requena (Requena, Valencia), Antoni Torres. Hospital Clínic (Barcelona), José Luis Viejo. Hospital General Yagüe (Burgos).