Differentiation syndrome (DS), also known by its former name of all-trans retinoic acid (ATRA) syndrome, is a potentially fatal complication in patients with acute promyelocytic leukemia (APL) on treatment with ATRA and/or arsenic trioxide (ATO).1 Its symptoms are nonspecific and can mimic other common complications in these patients, although dyspnea is the most frequent symptom.2 The incidence of DS is highly variable, primarily due to the absence of universally accepted diagnostic criteria and lack of specific biomarkers for detecting this complication.3 Clinical criteria include several “thoracic” criteria: dyspnea and the appearance of pulmonary infiltrates or effusions on chest radiograph. We report the case of a patient with APL in which chest computed tomography (CT) helped to promptly confirm the clinical suspicion of DS.
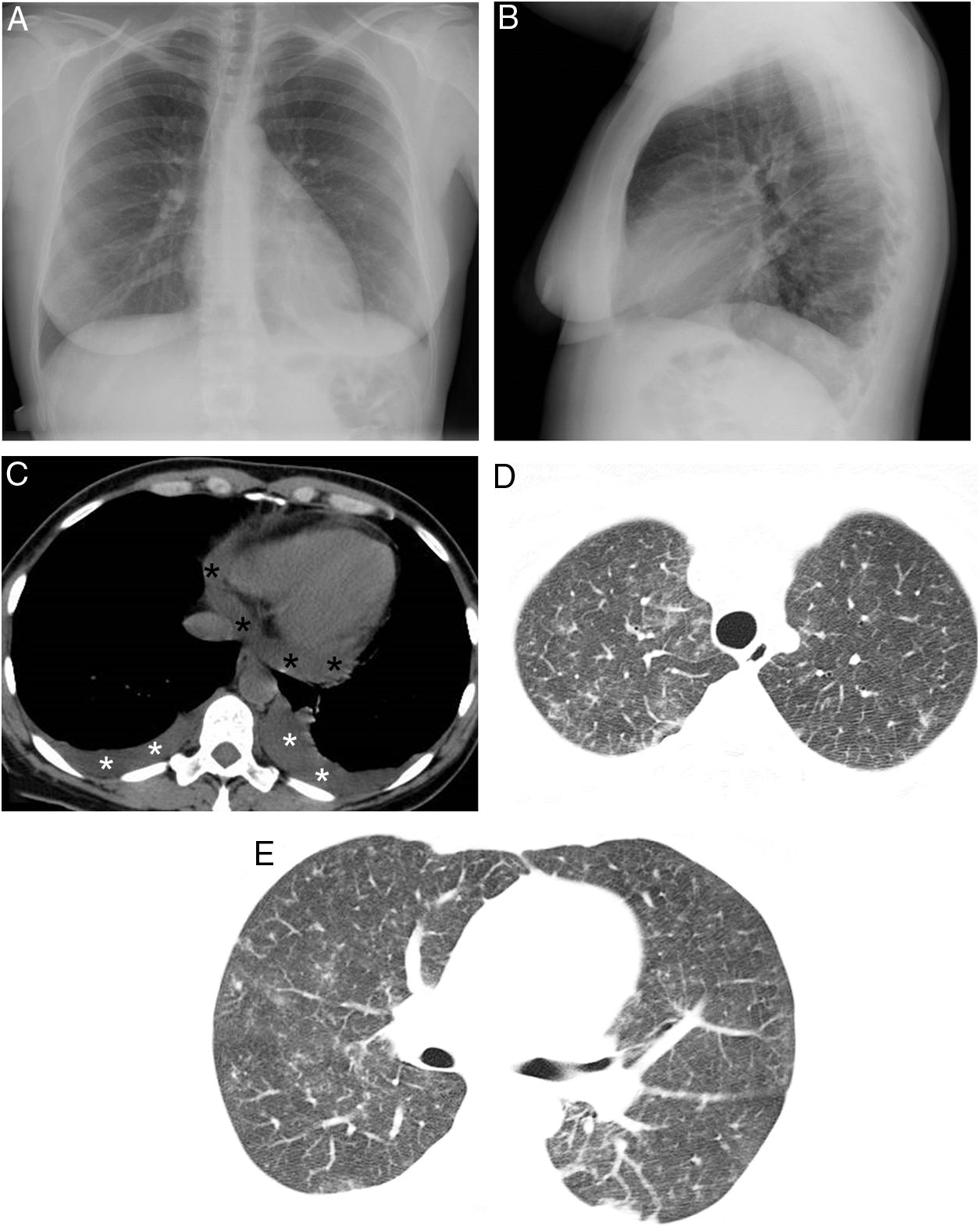
We present the case of a 48-year-old woman with a history of APL who experienced progressive dyspnea, myalgia, fever and weight gain 14 days after starting treatment with a combination of ATRA and ATO; DS was therefore suspected. Diagnosis of this syndrome requires fulfillment of at least 3 of the following criteria: dyspnea, fever (not explained by other causes), weight gain (>5kg), hypotension (unexplained), acute renal failure and appearance of parenchymal opacities or pleural or pericardial effusion on chest radiograph. Our patient met 2 clinical criteria (dyspnea and fever), as the weight gain was less than 5kg. At that time, chest radiograph showed a slight increase in the cardiothoracic ratio and the appearance of a left subpulmonic pleural effusion, with no parenchymal consolidations (Fig. 1A). Chest CT scan performed the same day confirmed the appearance of bilateral pleural effusion, pericardial effusion and several ground-glass opacities (Fig. 1B–D). Bronchoalveolar lavage with fiberoptic bronchoscopy (which ruled out both infection and pulmonary hemorrhage) and an echocardiogram (which ruled out heart failure) were also carried out. Thus, 3 diagnostic criteria were eventually fulfilled. The diagnosis of DS secondary to ATRA/ATO treatment was therefore made, and treatment was initiated immediately with corticosteroids (10mg i.v. dexamethasone/12h). Treatment with ATRA/ATO was suspended until the patient showed clinical improvement 5 days after initiating corticosteroid treatment, which was gradually withdrawn in the following days.
(A and B) Posteroanterior and lateral projections in which no parenchymal consolidations can be seen. A probable small left subpulmonic pleural effusion is observed, on noting an increased distance between the stomach and left lung base. (C) Axial chest CT scan (mediastinal window) showing new-onset bilateral pleural effusions (white asterisks) and pericardial effusion (black asterisks). (D and E) Axial chest CT scans (lung parenchyma window) in which new-onset bilateral ground-glass opacities are observed.
DS is a complication that can affect a varying proportion (2%–48%) of patients with APL on treatment with ATRA and/or ATO.1 This very diverse incidence is mainly due to the application of different diagnostic criteria, lack of specific biomarkers, and the use of variable induction regimens and preventive strategies.3 From a clinical point of view, physicians treating these patients should recognize the signs and symptoms of DS early on, and instigate the correct treatment (which usually involves the administration of corticosteroids and temporary suspension of ATRA/ATO).1 Unfortunately, the clinical presentation of DS is very nonspecific, and includes the following signs and symptoms4: dyspnea (most common symptom), hypotension, myalgia, fever, weight gain, acute renal failure, and appearance of peripheral edema or effusions (pleural, pericardial). These manifestations can be seen in many other clinical conditions that can complicate the course of hematology patients, such as fluid overload, opportunistic infections or pulmonary hemorrhage, among others.5 Laboratory data are also very nonspecific, the most common abnormalities in DS being: leukocytosis, coagulopathy and elevated liver enzymes and creatinine.5 The Montesinos diagnostic criteria (described above) are the most widely accepted at present.6 The presence of pulmonary opacities or effusion on chest radiograph is more frequent in patients with severe forms of DS, but their absence does not exclude the diagnosis of DS or other complications that may manifest radiologically in a similar manner.6–8 In our case, we decided to perform a chest CT early on, as the chest radiograph showed a suspicious pleural effusion and the patient met 2 of the Montesinos clinical criteria. This study revealed bilateral parenchymal opacities, bilateral pleural effusion and pericardial effusion (not visible in the radiograph), satisfying 3 clinical criteria to reach a firmer diagnosis of DS. We believe that early use of chest CT in suspicious or incipient cases of DS may be useful in the diagnostic and therapeutic management of patients with APL with possible DS secondary to treatment with ATRA/ATO, since this entity lacks universally accepted diagnostic criteria and specific biomarkers to confirm clinical suspicion.
Please cite this article as: Gorospe Sarasúa L, Ventura-Díaz S, Ayala-Carbonero AM, Gambí-Pisonero E, Sánchez-Tornero de la Cruz A, Pérez-Lamas L, et al. Síndrome de diferenciación en paciente con leucemia promielocítica aguda: importancia de la TC de tórax. Arch Bronconeumol. 2020;56:326–327.