To determine the general and specific utility in diagnosis and/or treatment of induced sputum (IS) inflammatory cell counts in routine clinical practice.
MethodsRetrospective study of 171 patients referred for clinical sputum induction over a 1-year period in the pulmonology department of a referral hospital. Independent observers established whether the information provided by IS inflammatory cell count was useful for making diagnostic and therapeutic decisions.
ResultsThe most frequent reasons for determination of IS inflammatory cell count were: asthma 103 (59.20%); uncontrolled asthma 34 (19.54%); chronic cough 19 (10.9%), and gastroesophageal reflux 15 (8.6%). In 115 patients (67.3%) it was generally useful for diagnosis and/or treatment; in 98 patients (57.3%) it provided diagnostic information and in 85 patients (49.7%) it assisted in therapeutic decision-making. In asthma, uncontrolled asthma, chronic cough and gastroesophageal reflux, the results were useful in 71.8%, 67.6%, 47.4% and 60%, respectively.
ConclusionThe information provided by IS inflammatory cell count is extremely useful in clinical practice, especially in asthma and chronic cough. These results may justify the inclusion of the IS technique in pulmonology departments and asthma units of referral centers.
Determinar la utilidad general y específica (diagnóstica y/o terapéutica) del recuento de las células inflamatorias (RCI) del esputo inducido (EI) en situación de asistencia clínica real.
MétodosEstudio retrospectivo que incluyó a los 171 pacientes que durante un año se les recogió un EI para determinar su RCI en un servicio de Neumología de un hospital de referencia. Observadores independientes al equipo médico habitual establecieron si la información proporcionada por el RCI del EI fue útil en la toma de decisiones diagnósticas y terapéuticas.
ResultadosLas causas más frecuentes que motivaron la solicitud del RCI del EI fueron: asma 103 (59,20%); asma de control difícil 34 (19,54%); tos crónica 19 (10,9%), y reflujo gastroesofágico 15 (8,6%). En 115 (67,3%) pacientes el RCI del EI resultó clínicamente útil (valoración general); en 98 (57,3%) proporcionó información diagnóstica, y en 85 (49,7%), información terapéutica relevante. En el asma, asma de control difícil, tos crónica y reflujo gastroesofágico fue útil en el 71,8, el 67,6, el 47,4 y el 60%, respectivamente.
ConclusionesLa información proporcionada por el RCI del EI resulta de gran utilidad en la práctica clínica, particularmente en el asma y la tos crónica. Estos resultados podrían proporcionar argumentos para recomendar la incorporación de la técnica en los servicios de Neumología de referencia y en las unidades de excelencia de asma.
Bronchial inflammation plays a major role in the pathogenesis of important respiratory tract diseases, such as asthma or chronic obstructive lung disease (COPD). A tool for evaluating the type and intensity of bronchial inflammation would be of great benefit in the assessment and control of these diseases, particularly in the more severe forms.
Endobronchial biopsy is the gold standard in the study of bronchial inflammation; being an invasive procedure, however, its use as a diagnostic tool is limited.1 Accordingly, interest is growing in non-invasive procedures, and new techniques for measuring fractional exhaled nitric oxide (FENO) and inflammatory cell count (ICC) in induced sputum (IS) are attracting particular attention.
While FENO identifies eosinophilic bronchial inflammation, it does not recognize other inflammatory types, so its utility is limited.2 IS is a validated standardized technique, considered the gold standard non-invasive methods for evaluating bronchial inflammation and for distinguishing between inflammatory phenotypes.3 Its clinical applications are becoming more refined, and it is of particular benefit in asthma, due to its high yield. It can be used as a complementary diagnostic tool in asthma,4 and is of benefit in determining inflammatory phenotypes,5 adjusting treatment, and predicting therapeutic response.6,7 It has also been indicated in the management of difficult-to-control asthma (DCA) in Spanish and international guidelines.8–10 Its use is not restricted purely to asthma: it is also useful for determining the etiology of chronic cough,11,12 gastroesophageal reflux (GER)13 and other entities, such as COPD, infectious diseases, eosinophilic bronchitis, lung cancer, interstitial lung diseases, and heart failure.14–19
However, despite its validity and applicability, ICC in IS is not routinely performed in standard clinical practice, probably because it requires a certain degree of technical experience in obtaining, manipulating and interpreting the samples, in addition to being laborious and costly. Nevertheless, the data provided by IS testing is so obviously of interest10 that we are surprised how rarely it is used in high-level pulmonology departments. The aim of this study was to evaluate the contribution of ICC in IS in healthcare practices.
MethodsDesignThis was a retrospective, descriptive study performed in standard clinical practice to determine the clinical utility of ICC in IS.
Study PopulationAll patients who underwent ICC in IS as part of their standard care in the pulmonology department of our hospital over the course of 1 year (May 2012–May 2013) were included, irrespective of their previous treatment, which often included inhaled corticosteroids (ICS), particularly in asthma patients. Patients who underwent the procedure for exclusively investigational purposes were excluded.
Ethical and Legal AspectsSince this was a descriptive, retrospective study performed in standard clinical practice conditions, the Clinical Research Ethics Committee was informed only of our interest in collecting this information from the clinical records of the patients. All study data collection was anonymous.
Primary EndpointsThe primary endpoint was the proportion of patients in whom IS was clinically useful. IS was considered useful when the ICC provided information that could be used for establishing a diagnosis and/or when it led directly to a decision on therapeutic management. Three observers, independent from the treating physicians (SB, LS, and GC), assessed these premises by reviewing the clinical records.
The asthma group included patients with clinically suspected asthma and those with a previous diagnosis of asthma. In the DCA group, all patients had asthma meeting criteria for poor control. IS was considered of use in diagnosis when the determination of bronchial eosinophilia led to the diagnosis of patients with a clinical history consistent with asthma.20 It was also classified as useful in patients with a previously established diagnosis of asthma or DCA, when the determination of the inflammatory phenotype helped clarify the nature of the patient's respiratory symptoms9,10,21 in the following circumstances: suspicion of poor treatment compliance, exposure to airborne allergens, or insufficient treatment in the case of an eosinophilic phenotype; suspicion of an erroneous diagnosis or another associated disease or resistance to corticosteroids in the case of bronchial neutrophilia; a paucigranulocytic phenotype suggested controlled eosinophilia, confounding diagnoses, or paucigranulocytic variables. IS was considered therapeutically useful in the following situations: in patients with bronchial eosinophilia when the decision was taken to increase ICS, initiate systemic corticosteroids (SCS), initiate interventions for improving compliance, or initiate leukotriene receptor antagonists;6,7 in patients with a neutrophilic phenotype6,7 when antibiotics or long-acting β2-agonists were initiated or ICS dosing was reduced; and in patients with a paucigranulocytic phenotype when the addition of long-acting β2-agonists was evaluated or the steroid dose was reduced.6,7
In patients with chronic cough, ICC was considered to be useful when findings helped identify the reason for the cough11,12 in the following circumstances: a case of bronchial eosinophilia arousing suspicion of asthma, eosinophilic bronchitis, or occupational disease; or a neutrophilic phenotype aroused suspicion of infectious bronchitis or bronchiectasis. IS was considered of therapeutic utility when findings led to the initiation of ICS or antibiotic therapy. Finally, the finding of lipophages in patients with chronic cough or clinically suspected GER helped identify a diagnosis of GER. Initiation of antacids or antireflux measures was considered a therapeutic contribution.
Secondary EndpointsDemographic, clinical and functional data were collected from all patients, and the main reasons for requesting IS and the characteristics of the sample (cell count, sample quality and inflammatory phenotype) were recorded.
ProceduresSpirometrySpirometry was performed using a Daptospir-600 device (Sibelmed SA, Barcelona, Spain), by an experienced operator, following SEPAR 2013 guidelines.22 The reference values were those established for a Mediterranean population.23
Fractional Exhaled Nitric OxideThis was carried out using an electrochemical device (NO Vario Analyzer. FILT Lungen and Thorax Diagnostic GmHb, Berlin, Germany) at a flow of 50ml/s, following the recommendations of the ATS/ERS.24 A significant elevation was considered any value ≥50ppb.25
Induced SputumThe process of inducing and processing sputum was performed by specially trained and qualified healthcare professionals, according to the standard procedure.26 Briefly, the sample was obtained after nebulization with hypertonic saline solution 3% using an ultrasound nebulizer (Omron NEU07). Serial spirometries were performed throughout induction. Procedures were suspended if FEV1 fell by 20% or more from baseline. The sample was processed within 2h of collection. Mucoid cumulates were selected manually, separated from the saliva, and treated with dithiothreitol (Sputolysin®, Calbiochem Corp., San Diego, CA) diluted 1:10. Dithiothreitol was added to a volume equivalent to 4 times the weight in milligrams of the selected plugs, along with the same volume of phosphate-buffered saline solution. Cell viability (live cells), concentration (cells/gram of sputum) and the percentage of squamous cells were evaluated (the latter considered as upper airway contamination), using hemocytometry and trypan blue staining. The cells were centrifuged to obtain a sediment that was used to determine the differential leukocyte count, using Wright-Giemsa staining, according to the procedure described by Pizzichini et al.27 Lipophages were also identified, using Oil Red O lipid staining.28 The sample was classified as “high quality” when the cell concentration was >1×106cells/g, viability>40%, and a concentration of epithelial cells<20%. IS samples were classified according to the differential leukocyte count into 4 inflammatory phenotypes, according to the criteria of Simpson et al.29: eosinophilic (eosinophils>3%); neutrophilic (neutrophils>61%); mixed (eosinophils>3% and neutrophils>61%), and paucigranulocytic (eosinophils<3% and neutrophils<61%).2,30
Asthma Control TestA validated version in Spanish31 of the Asthma Control Test was used to establish the degree of clinical control in asthma patients. This is a 5-item patient-reported outcome measure of the degree of control of asthma in the last 4 weeks. Answers to each question are scored individually from 1 to 5 points, for a total score ranging from 5 (worst control possible) to 25 (best control possible). A score of 20 points or more is considered “controlled asthma” and 19 points or fewer is “uncontrolled asthma”. Uncontrolled asthma is subdivided into “partially controlled asthma” (16–19 points) and “poorly controlled asthma” (5–15 points).
Statistical AnalysisResults are expressed as means and standard deviation for continuous variables with normal distribution. For continuous variables which did not fit a normal distribution, values were expressed in median and interquartile range, and for categorical variables, frequency and percentages were presented. Analysis of variance (ANOVA) or the Kruskal–Wallis test were used to compare demographic and clinical data among the 4 groups, depending on whether distribution of variables was normal or not. Categorical variables were compared using the Chi-squared test. Results were considered significant in case of P<.05. All analyses were carried out using the SPSS statistical package (v.22).
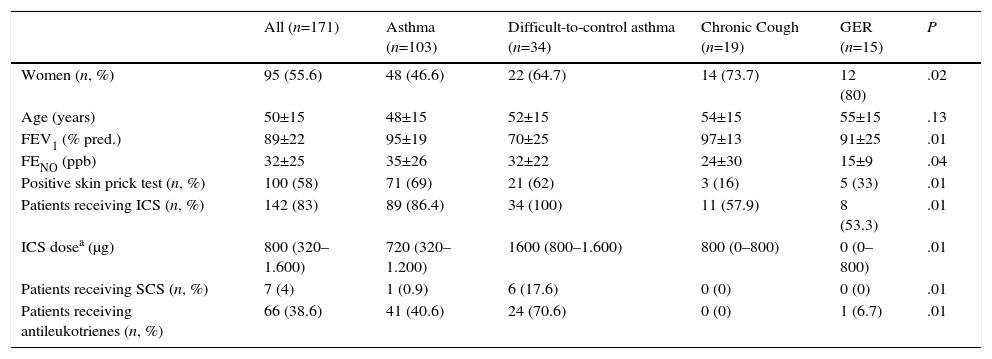
ResultsA total of 171 patients were included, 95 (55.6%) of whom were women, and mean age was 50 (±15) years. Sociodemographic and clinical data of the study group are shown in Table 1.
Sociodemographic and Clinical Characteristics of the Overall Study Series and by Subgroups According to the Reason for Requesting Inflammatory Cell Count in Induced Sputum.
| All (n=171) | Asthma (n=103) | Difficult-to-control asthma (n=34) | Chronic Cough (n=19) | GER (n=15) | P | |
|---|---|---|---|---|---|---|
| Women (n, %) | 95 (55.6) | 48 (46.6) | 22 (64.7) | 14 (73.7) | 12 (80) | .02 |
| Age (years) | 50±15 | 48±15 | 52±15 | 54±15 | 55±15 | .13 |
| FEV1 (% pred.) | 89±22 | 95±19 | 70±25 | 97±13 | 91±25 | .01 |
| FENO (ppb) | 32±25 | 35±26 | 32±22 | 24±30 | 15±9 | .04 |
| Positive skin prick test (n, %) | 100 (58) | 71 (69) | 21 (62) | 3 (16) | 5 (33) | .01 |
| Patients receiving ICS (n, %) | 142 (83) | 89 (86.4) | 34 (100) | 11 (57.9) | 8 (53.3) | .01 |
| ICS dosea (μg) | 800 (320–1.600) | 720 (320–1.200) | 1600 (800–1.600) | 800 (0–800) | 0 (0–800) | .01 |
| Patients receiving SCS (n, %) | 7 (4) | 1 (0.9) | 6 (17.6) | 0 (0) | 0 (0) | .01 |
| Patients receiving antileukotrienes (n, %) | 66 (38.6) | 41 (40.6) | 24 (70.6) | 0 (0) | 1 (6.7) | .01 |
FENO, fractional expired nitric oxide; FEV1, forced expiratory volume in 1s; GER, gastroesophageal reflux; ICS, inhaled corticosteroid; pred., predicted value; SCS, systemic corticosteroid.
Values expressed as mean±standard deviation, median (interquartile range) or as percentage, as indicated.
Patients included in the study were classified according to the reason for ordering an IS. The most common reasons were asthma in general (103 [59.20%]) and DCA (34 [19.54%]), to complete the diagnostic process or to determine the inflammatory phenotype. The next most common reasons were chronic cough (19 [10.9%]) and suspected GER (15 [8.6%]). Three cases (1.7%) were classified as “other causes” and were excluded because of their heterogenic nature.
Patients were classified according to the severity of their asthma and DCA, as follows: intermittent asthma 10 (7.2%) cases; mild persistent asthma 41 (29.9%), moderate 33 (24.1%) and severe 45 (32.8%). Eight cases (5.8%) were being investigated for suspected asthma. The mean Asthma Control Test score in the asthma group was 21.36 (±5.10) and in the DCA group, it was 15.9 (±5.12).
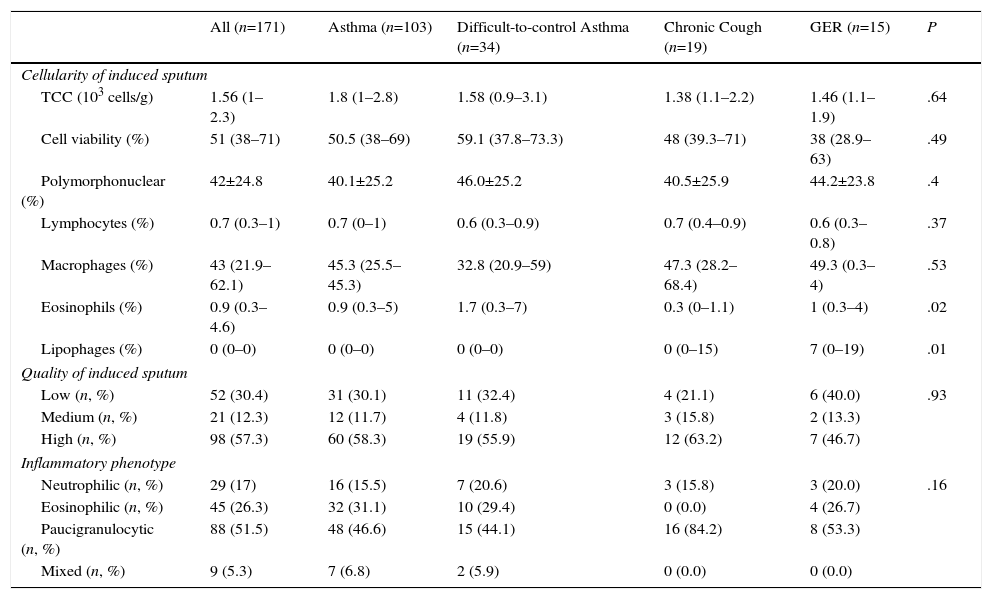
Table 2 shows the characteristics of the IS samples. In the ICC, the highest percentage of eosinophils was observed in the DCA group, and the highest lipophage percentage was in patients with chronic cough and GER. IS was classified as “high quality” in 98 (57.3%) cases. The paucigranulocytic phenotype was predominant among the overall study cohort (88 [51.5%]) and among the subgroups for which IS was requested, although the eosinophilic phenotype emerged as the second most common phenotype in the asthma and DCA groups with 32 (31.1%) and 10 (29.4%) cases, respectively.
Characteristics of Induced Sputum.
| All (n=171) | Asthma (n=103) | Difficult-to-control Asthma (n=34) | Chronic Cough (n=19) | GER (n=15) | P | |
|---|---|---|---|---|---|---|
| Cellularity of induced sputum | ||||||
| TCC (103 cells/g) | 1.56 (1–2.3) | 1.8 (1–2.8) | 1.58 (0.9–3.1) | 1.38 (1.1–2.2) | 1.46 (1.1–1.9) | .64 |
| Cell viability (%) | 51 (38–71) | 50.5 (38–69) | 59.1 (37.8–73.3) | 48 (39.3–71) | 38 (28.9–63) | .49 |
| Polymorphonuclear (%) | 42±24.8 | 40.1±25.2 | 46.0±25.2 | 40.5±25.9 | 44.2±23.8 | .4 |
| Lymphocytes (%) | 0.7 (0.3–1) | 0.7 (0–1) | 0.6 (0.3–0.9) | 0.7 (0.4–0.9) | 0.6 (0.3–0.8) | .37 |
| Macrophages (%) | 43 (21.9–62.1) | 45.3 (25.5–45.3) | 32.8 (20.9–59) | 47.3 (28.2–68.4) | 49.3 (0.3–4) | .53 |
| Eosinophils (%) | 0.9 (0.3–4.6) | 0.9 (0.3–5) | 1.7 (0.3–7) | 0.3 (0–1.1) | 1 (0.3–4) | .02 |
| Lipophages (%) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–15) | 7 (0–19) | .01 |
| Quality of induced sputum | ||||||
| Low (n, %) | 52 (30.4) | 31 (30.1) | 11 (32.4) | 4 (21.1) | 6 (40.0) | .93 |
| Medium (n, %) | 21 (12.3) | 12 (11.7) | 4 (11.8) | 3 (15.8) | 2 (13.3) | |
| High (n, %) | 98 (57.3) | 60 (58.3) | 19 (55.9) | 12 (63.2) | 7 (46.7) | |
| Inflammatory phenotype | ||||||
| Neutrophilic (n, %) | 29 (17) | 16 (15.5) | 7 (20.6) | 3 (15.8) | 3 (20.0) | .16 |
| Eosinophilic (n, %) | 45 (26.3) | 32 (31.1) | 10 (29.4) | 0 (0.0) | 4 (26.7) | |
| Paucigranulocytic (n, %) | 88 (51.5) | 48 (46.6) | 15 (44.1) | 16 (84.2) | 8 (53.3) | |
| Mixed (n, %) | 9 (5.3) | 7 (6.8) | 2 (5.9) | 0 (0.0) | 0 (0.0) | |
GER, gastroesophageal reflux; IS, induced sputum; TCC, total cell count.
Values expressed as mean±standard deviation, median (interquartile range) or as percentage, as indicated.
A weakly positive correlation was observed between age and neutrophilia in ICC (R=0.15, P=.05). However, no correlation was observed between the presence of eosinophils in IS and FENO values (R=0.12, P=.14).
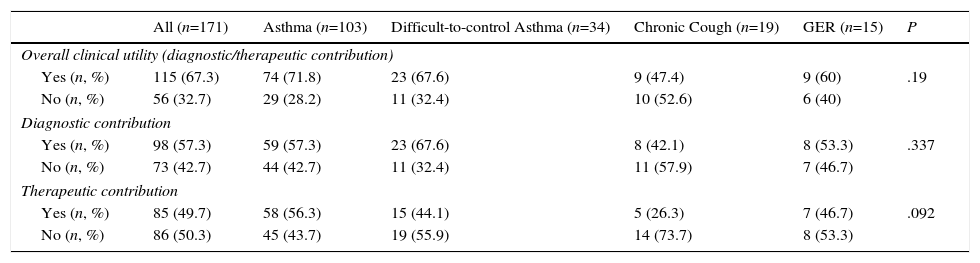
Finally, the IS result contributed to clinical decision-making in 115 (67.3%) of cases, assisting diagnosis in 98 (57.3%) patients, and therapeutic decisions in 85 (49.7%). The results are listed by reason for IC in Table 3. IS was of greatest benefit in decision-making in the general asthma group, followed by DCA: 74 (71.8%) and 23 (67.6%), respectively, although there were no differences in its utility among the different reasons for request. In the asthma group, a similar contribution to the diagnostic process (59 [57.3%]) and to therapeutic decision-making (58 [56.3%] was also observed. However, in the DCA group, IS provided more useful information for diagnostic (23 [57.6%] patients) than therapeutic (15 [44.1%] patients) decision-making. Likewise, in the chronic cough group, IS was more useful for guiding diagnosis (Table 3). In patients with suspected GER, the identification of a significant number of lipophages supported the diagnosis in 8 (53.3%) patients.
Contribution of Results Provided by Inflammatory Cell Count in Induced Sputum in Overall Clinical, Diagnostic and Therapeutic Decision-making.
| All (n=171) | Asthma (n=103) | Difficult-to-control Asthma (n=34) | Chronic Cough (n=19) | GER (n=15) | P | |
|---|---|---|---|---|---|---|
| Overall clinical utility (diagnostic/therapeutic contribution) | ||||||
| Yes (n, %) | 115 (67.3) | 74 (71.8) | 23 (67.6) | 9 (47.4) | 9 (60) | .19 |
| No (n, %) | 56 (32.7) | 29 (28.2) | 11 (32.4) | 10 (52.6) | 6 (40) | |
| Diagnostic contribution | ||||||
| Yes (n, %) | 98 (57.3) | 59 (57.3) | 23 (67.6) | 8 (42.1) | 8 (53.3) | .337 |
| No (n, %) | 73 (42.7) | 44 (42.7) | 11 (32.4) | 11 (57.9) | 7 (46.7) | |
| Therapeutic contribution | ||||||
| Yes (n, %) | 85 (49.7) | 58 (56.3) | 15 (44.1) | 5 (26.3) | 7 (46.7) | .092 |
| No (n, %) | 86 (50.3) | 45 (43.7) | 19 (55.9) | 14 (73.7) | 8 (53.3) | |
GER, gastroesophageal reflux.
Values expressed as absolute frequencies and percentages.
The main finding of our study is that ICC in IS is useful in routine clinical practice, since it provides important information for clinical decision-making in two thirds (67.3%) of patients in whom this complementary procedure was requested.
Most experience and data on the use of IS are from asthma patients. Accordingly, IS was usually ordered in the context of asthma and DCA. Diagnostic applications are based mainly on the well-established relationships between asthma and eosinophilia in sputum.5 If a cutoff point is set at 1% eosinophils in sputum, IS has a sensitivity of 80% and a specificity of 95% for confirming a diagnosis of asthma.20 Although the most typical finding is eosinophilia in sputum, a systematic review of over 25 studies in asthma patients from different populations with varying degrees of severity,32 and more recently, a study which analyzed 508 patients with asthma,33 found that approximately 50% of these patients had non-eosinophilic asthma. In our series, more patients had non-eosinophilic asthma than generally described in the literature. However, the contribution of IS to confirming the diagnosis or determining the inflammatory phenotype was 67.6% in DCA and 57.3% in asthma.
Characterization of the inflammatory phenotype has important therapeutic implications, since each has a different response to anti-inflammatory treatment. Little et al.34 observed that patients with asthma who showed eosinophilia in IS had a significant increase in FEV1 after 2 weeks of treatment with SCS. Moreover, IS tests can reduce the number of exacerbations in asthmatic patients. Two randomized trials6,7 conducted in asthmatics whose treatment was adjusted according to clinical practice guideline recommendations or according to their IS eosinophil count showed a reduction in the number of exacerbations in patients whose treatment was established on the basis of their ICC. These results were confirmed in a recent systematic review,35 and are reflected in the latest ATS/ERS guidelines on the management of severe asthma.24 These recommendations state that in hospitals with the appropriate experience, treatment of patients with severe asthma should be based on both clinical criteria and monitoring of IS eosinophil levels. We also studied the contribution of IS to therapeutic decision-making in asthma patients. Results centered on the eosinophilic and neutrophilic phenotypes, but in some cases the decision to reduce steroid treatment was made in clinically stable patients who revealed a paucigranulocytic phenotype, according to clinical practice guidelines. Nevertheless, these premises only apply to selected patients, since the monitoring of eosinophilia in IS has not been adopted as a routine method in standard clinical practice.
In patients with chronic cough, defined as a >3-week history of cough not immediately preceded by an acute process, IS is recommended to complete the diagnostic process.11,12 Bronchial eosinophilia revealed in patients with chronic cough can help guide the diagnosis of asthma, eosinophilic bronchitis, or occupational asthma, and can also predict a favorable response to steroid treatment. In our study, none of our chronic cough patients had eosinophilia in IS. However, in 3 (15.8%) patients, neutrophilia predominated, suggesting infectious disease. The finding of lipophages in IS (>7%) is 90% sensitive and 89% specific for a diagnosis of GER.13 Lipophages (>7%) were found in 8 study patients investigated for chronic cough and for suspected GER, which explained 42.1% of causes of chronic cough, and supported 53.3% of suspected GER cases.
In line with the previous literature,21,36 we found evidence among our series of a trend toward increased bronchial neutrophilia as age advances. However, more studies are need to determine if these findings affect the diagnostic yield of IS in elderly patients.
In spite of all the reports in the literature describing the benefits of IS, only Moritz et al.,37 in a series of 151 IS samples, found the results to be clinically useful in standard clinical practice, because they modified medical treatment in 82 (55%) of cases. The contribution was even greater in asthmatic patients: steroid dosing was modified in 48 patients (64.7%). The results of our study are similar to those described by Moritz et al. However, in our opinion, the contribution of the procedure to the diagnostic process must be included if the real benefit is to be studied.
In spite of the limitations of the retrospective nature of this study (which, for example, in the case of asthma made it impossible to determine any improvements in future risk), the utility of the procedure was evaluated by observers who were independent from the treating physicians, in order to reduce subjectivity bias. Moreover, IS samples were induced, processed and evaluated according to a systematized protocol, by highly experienced technical staff, in compliance with the strict quality control standards of our laboratory. These precautions contributed to reducing potential variability among observers assessing ICC and standardizing sample collection and processing.
To conclude, our study demonstrates that ICC in IS is beneficial in clinical decision-making, particularly in 2 common entities, asthma and chronic cough. We provide arguments for recommending the use of this technique in clinical practice, at least in referral pulmonology departments, particularly those equipped with dedicated asthma units.
Conflict of InterestsThe authors declare that they have no conflict of interests.
Please cite this article as: Barril S, Sebastián L, Cotta G, Crespo A, Mateus E, Torrejón M, et al. Utilidad del esputo inducido en la práctica clínica habitual. Arch Bronconeumol. 2016;52:250–255.