Inflammatory pseudotumors are a relatively uncommon pathology of uncertain etiology, generally considered to be reactive in origin. They may be observed in different locations as single or multiple masses. One of the possible forms of presentation is intrapulmonary.
Despite its low frequency, this pathology should be considered in the differential diagnosis of lung nodules, even though the histologic results and the imaging tests can be confusing. In addition, pulmonary inflammatory pseudotumors present a low malignancy with good response to surgical treatment as well as to pharmacological therapy, although to a lesser degree.
We present a bibliographic review of this pathology based on two cases observed in our hospital. Both patients debuted with non-specific respiratory symptoms and lung nodules on imaging studies that were suspicious for neoplastic processes. After an exhaustive study, the diagnosis of pulmonary inflammatory pseudotumor was reached, with excellent responses to the treatment used in each case.
El seudotumor inflamatorio es una patología relativamente poco frecuente de etiología incierta, generalmente considerada de origen reactivo. Puede observarse en diferentes localizaciones como masas únicas o múltiples. La intrapulmonar es una de sus posibles formas de presentación.
A pesar de su baja frecuencia, esta patología debe tenerse en cuenta a la hora de realizar el diagnóstico diferencial de nódulos pulmonares. De otro modo, los resultados histológicos y de las pruebas de imagen pueden llegar a ser confusos. Además, presenta una baja malignidad con buena respuesta al tratamiento quirúrgico y en menor medida al farmacológico.
Presentamos una revisión bibliográfica de esta patología basándonos en dos casos observados en nuestro hospital. Ambos pacientes comenzaron con cuadros respiratorios inespecíficos y nódulos pulmonares en pruebas de imagen, sospechosos de proceso neoplásico. Tras su estudio exhaustivo se llegó al diagnóstico de seudotumor inflamatorio pulmonar con excelente respuesta al tratamiento usado en cada caso.
Inflammatory pseudotumor (IP) is an uncommon pathology of unknown etiology, although it has been associated with multiple factors.1,2 It is considered a generally reactive process that is characterized by irregular growth of inflammatory cells: myofibroblasts, plasma cells, macrophages, histiocytes, etc.1,3,4
The presentation and natural history of IP are very variable, and they may appear in the mesentery, retroperitoneal space, oropharynx, mucosa, etc. They are not frequently located in the thorax and are present in 0.04%–1.2% of thoracotomies, while representing 0.7% of lung tumors.1,5,6
IP may be a confounding factor in the differential diagnosis of lung masses. In addition, early diagnosis benefits patients as the prognosis worsens with the progression of symptoms. We present two cases that represent the most common forms of presentation of this pathology, which are completely different from each other with regards to their characteristics and are initially managed like neoplasms.
Clinical ObservationCase 1A 54-year-old woman came to the emergency department due to symptoms that had been evolving over several days, including cough, rusty sputum, fever, thoracic pain, and progressive dyspnea on exertion. She reported having had frequent lower respiratory tract processes in recent years. Physical examination revealed rhonchus and bibasilar crackles on lung auscultation. Hemogram showed leukocytosis at 14.6×109l (normal range 4.5–10.5×109l) with neutrophilia. During hospitalization, the patient was diagnosed with previously unknown chronic hepatopathy due to B virus, with type B Child-Pugh cirrhosis.
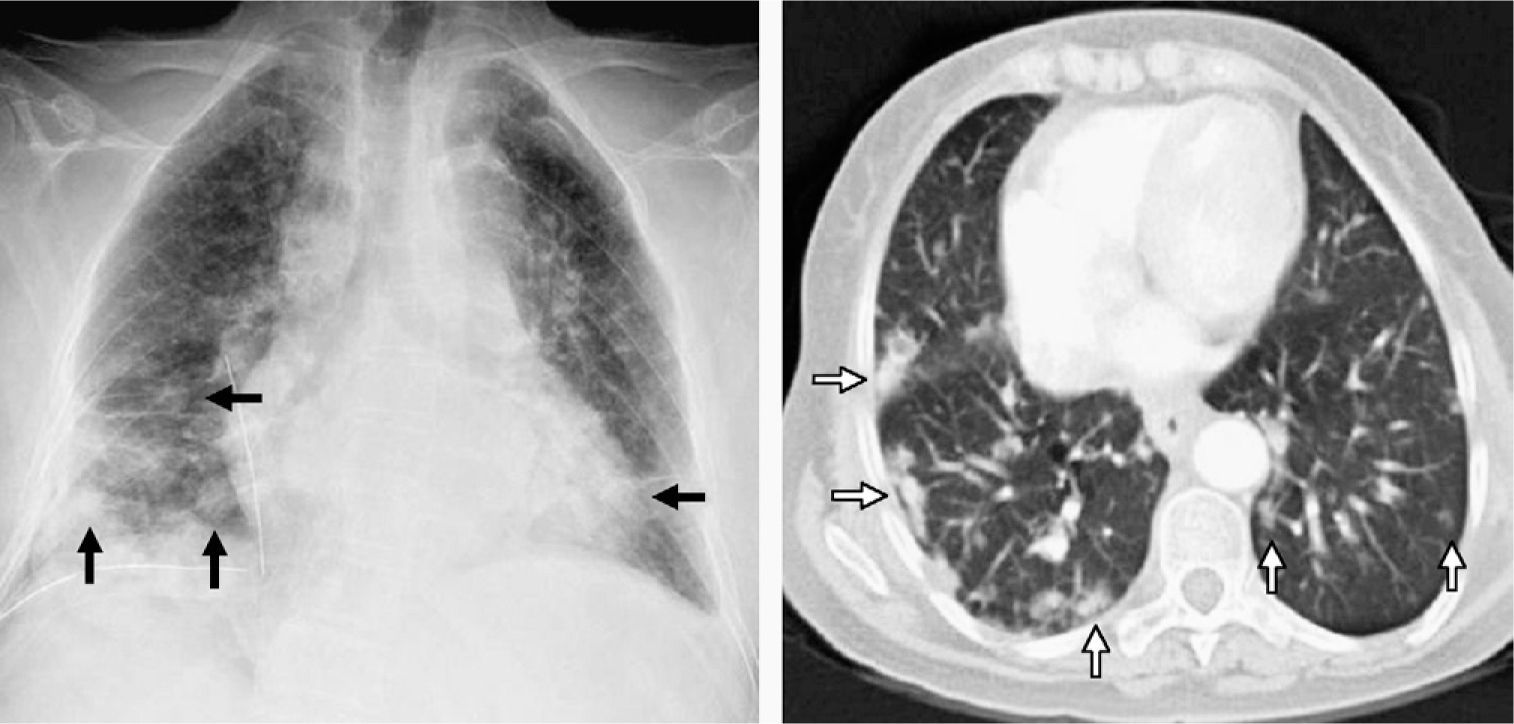
Given the suspicion of a pneumonic process, a plain chest X-ray was ordered (Fig. 1), which demonstrated multiple bibasilar nodules that were more numerous on the right side. A condensation was also observed in the right middle lobe, suggestive of a pneumonic process. With antibiotic treatment and respiratory physiotherapy, the patient improved.
On the left, chest radiography after surgical biopsy revealing multiple bibasilar nodules predominantly on the right side (black arrows). On the right, computed tomography (CT) axial cut with pulmonary window showing bibasilar peripheral hyperdense nodules with poorly outlined edges that are larger in size in the right hemithorax (white arrows).
Computed tomography (CT) was scheduled (Fig. 1, right), which confirmed multiple, well-defined, non-spiculated lung nodules, diffusely distributed in both lobes with basal and peripheral predominance. Subcarinal, peribronchial, and retroperitoneal lymphadenopathies with pathological characteristics were also observed. CT also confirmed opacity with air bronchograms in the right middle lobe with pneumonic characteristics. The suspected diagnosis was hematogenous metastasis of an unknown primary tumor versus a lymphoproliferative process.
Bronchoscopy did not demonstrate any intrabronchial lesions. Transbronchial biopsy came back with a result for polymorphous, lymphoid infiltrate, without ruling out a lymphoproliferative process. Surgical biopsy showed a heterogenous lymphoid infiltrate with no histologic signs of malignancy compatible with multifocal IP. A sample was sent to the National Oncological Research Center (Centro Nacional de Investigaciones Oncológicas–CNIO), which confirmed the diagnosis.
Given the bilaterality and extension of the nodules, treatment was started with steroid therapy. Good response was observed, with complete regression of the lesions at a check-up 8 months after the treatment had begun. The patient continued to be lesion-free until her death 7 months later due to complications associated with the cirrhosis.
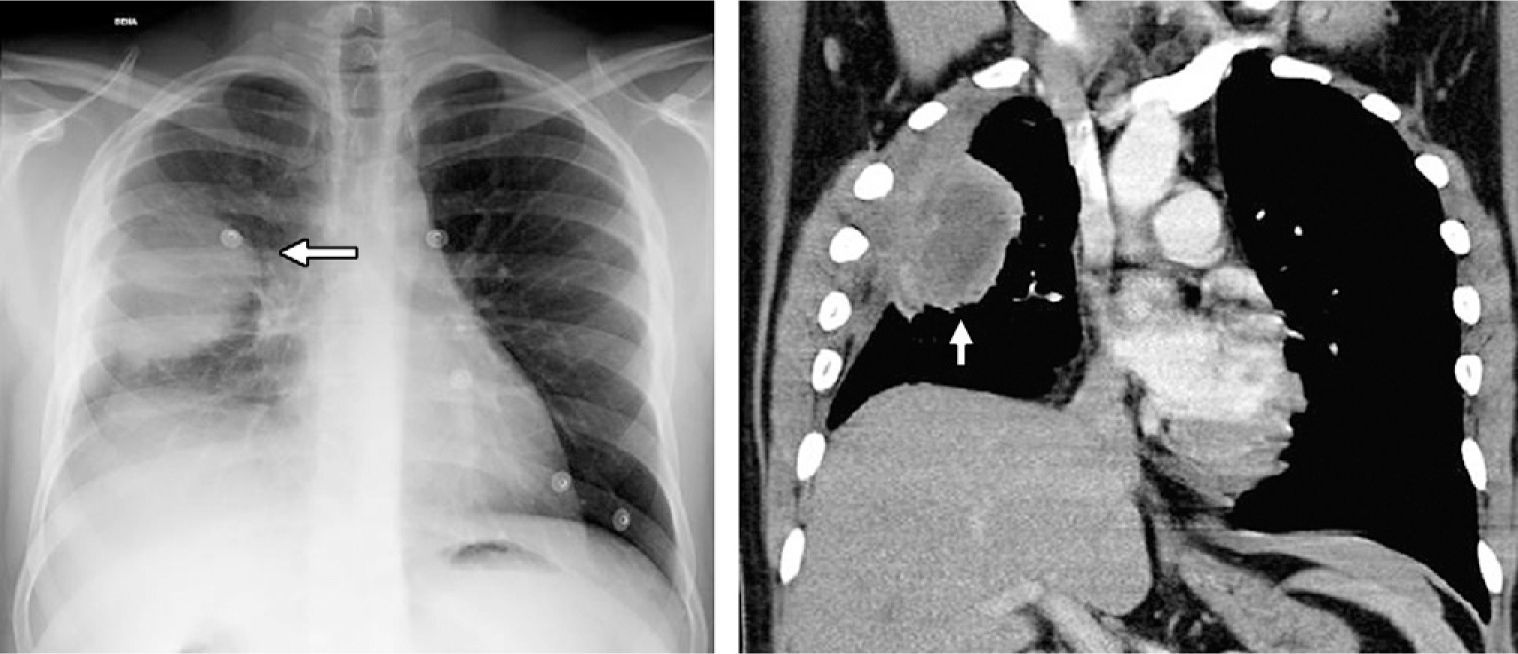
Case 2A 25-year-old male came to our emergency department due to oppressive pain in the right hemithorax that increased with exercise and episodes of self-limiting cough. He presented no personal history of interest. Clinical exploration and work-up were anodyne, except for a leukocyte count at the upper limit of normal without neutrophilia. Emergency chest radiography (Fig. 2) showed a homogenous right lung mass with well-defined edges in the upper right lobe that was in contact with the pleura and associated with the elevation of the ipsilateral hemidiaphragm.
On the left, chest radiography presenting a homogenous lung mass with well-defined edges in the upper right lobe (white arrow), in contact with the pleura and associated with the loss in volume. On the right, in the computed tomography (CT) coronal reconstruction with soft-tissue window, the well-defined mass can be observed with areas of central necrosis in contact with the pleura and producing thickening, as well as the loss in volume of the right hemithorax.
Chest CT (Fig. 2, right) revealed a mass in the right upper and middle lobes, measuring 60×43mm with partially defined edges and a central area of necrosis that was in contact with both scissures and pleura, which showed thickening. There was a loss in volume in the right hemithorax, and pathological right hilar and mediastinal lymphadenopathies were observed. A CT-guided biopsy obtained a sample with areas of fibrosis with plasma cells and eosinophils that were negative for malignancy. Given the uncertain diagnosis, it was decided to remove the mass surgically by transsegmental resection.
The anatomic pathology of the post-surgical piece showed no evidence of malignancy, although fibrosis and intense chronic inflammation were observed with some organizing pneumonia and follicular bronchiolitis-type changes. The definitive diagnosis was monofocal IP. During follow-up, the patient presented a positive evolution, with no signs of relapse to date (24 months after surgery).
DiscussionIP is a rare disease that is still being studied.6–8 It has been called many different things: inflammatory myofibroblastic tumor, plasma cell granuloma, fibroxanthoma, pseudosarcomatous fibromyxoid tumor and inflammatory myofibrohistiocytic proliferation.3,4,6,9,10 It is not considered a malignant process, although this data is controversial given its potentially invasive nature.3,7,8 Cases have been reported of aggressive behavior with vascular invasion, infiltration of adjacent structures, bone destruction, recurrence after surgery, metastatic dissemination or even sarcomatous degeneration.1,6,7,9 Some authors consider it a low-grade neoplasm.8,9
The etiology of IP is not precisely known. It has been related with a chronic, focal, uncontrolled inflammatory response to antigens or pulmonary infections.1,2,6,9 There are three histologic patterns of presentation, although it usually presents with a combination of the three with one predominant type: vascular myxoid, compact fusiform cell or hypocellular fibrous, with no clinical, radiological or prognostic differences amongst them.7,10
Pulmonary IP can be seen in a wide age range, with no predominance of sex. It is, nevertheless, more frequent in children and young adults (60% of cases are under the age of 40).1,3,5,7,11 It is the most frequent primary lung tumor in children,3,5,6,11 and its invasive behavior is more frequent in childhood (some 20% of cases).6
In 50%–70% of cases, IP is asymptomatic and it is accidentally discovered during imaging tests.4,5,7,9 Occasionally, the patients may report respiratory symptoms, such as dyspnea, thoracic pain, cough or hemoptysis related to the location of the IP, and in some cases fever or weight loss.1,2,9,11 Up to one-third of patients report a previous history of lower respiratory tract infections, but a clear chronology has not been shown.2,3,7,9,11 The laboratory tests are normal or have non-specific alterations.9
On imaging tests, variable characteristics may be observed. They usually appear in peripheral locations and in the lower lobes, and on rare occasion they are intrabronchial (10.7%–12%).3,4,9,11 They may be multifocal, but the most frequent presentation is as a single mass (up to 87%).3,7–9,11 Simple thoracic radiography usually shows round or oval lesions with well-defined edges and homogeneous density (coin lesion).2,5,11
With CT, the findings are variable and non-specific. Usually, well-defined, smooth-edged or lobulated masses are observed, with an enhanced heterogeneous pattern when contrast material is introduced.3,4,8,9,11 Sometimes, an enhanced pattern with irregular peripheral predominance has been described.3 Less frequently there are calcifications (15%, more frequent in children), necrosis (10%), airway affectation (10%, frequently a polyp), cavitation (5%), central scarring and, rarely, lymphadenopathies (7%) or hemorrhage.2–4,9,11
When using positron emission tomography (PET), pulmonary IP usually presents capitation with tumor characteristics, representing a false positive.6,12 This characteristic could be useful for ruling out relapses or distant metastases once the histologic diagnosis is confirmed, but there are no studies to this end. On magnetic resonance (MR), it presents an intermediate signal in T1-weighted sequences, hyperintensity in T2 and heterogeneous enhancement pattern after the administration of contrast, although we have not found thorough studies on IP with this technique.3,8
The final diagnosis is usually surgical given the lack of specificity of the findings and the difficulty to make a diagnosis by means of transbronchial or transthoracic biopsy.1,7,9 In the differential diagnosis, we should include malignant lung neoplasm and metastasis, as well as granuloma and hamartoma (especially when it presents with calcifications), hemangioma, adenoma, and pulmonary sequestration. In the case of endobronchial IP, we should include carcinoid, mucoepidermoid carcinoma, and adenoid cystic carcinoma.2,3,11
The first-choice treatment is surgical resection of the lesions with an adequate margin of safety,1,2,6–8,10 usually lobectomy/pneumonectomy in the central locations and segmental resection in the periphery. In invasive cases, more aggressive surgery is indicated.1,6,7 The prognosis is excellent, with 78%–100% of complete remission after 3.3 years and up to 89% at 10 years.6,7 Intrathoracic relapse has been reported in 5% of cases, usually related with affected margins. The risk factors related with poor evolution are the need for repeated surgery, with a reported 96% 5-year mortality, and a size larger than 3cm.6–8 There are reports of cases with extrathoracic relapse.
Steroid therapy presents varying effects, from complete resolution to utter ineffectiveness.6–9 There are several cases with good results when it is used as a primary treatment, although we have not found data about long-term follow-up.6 Thus, it may be an alternative treatment when there are contraindications for surgery (due to inoperability or number of lesions).2,6–8 In our first case report, there were 7 months of disease-free evolution before the patient died due to other causes. Chemotherapy presents varying results, with few studies and unfavorable results alone. It could be useful in multifocal lesions, local invasion or relapse.6,7 Radiotherapy presents inconclusive results and has not been widely used.6,7 There have also been reports of rare cases of spontaneous regression.2,9
Please cite this article as: Fornell-Pérez R, et al. Dos formas de presentación del seudotumor inflamatorio pulmonar. Arch Bronconeumol. 2012; 48:296-9.