Methotrexate (MTX) is used to treat cancers, several forms of arthritis and other rheumatic conditions, although MTX may cause pulmonary toxicity related to the production of free oxygen radicals, various cytokines. Infliximab (IB) with its potent effect on tumor necrosis factor-alpha (TNF-α) inhibition also inhibits the release of endothelin-1 (ET-1). We aimed to investigate whether IB reduces pulmonary damage induced by an overdose of MTX.
MethodThe rats were divided into 3 groups of 8 animals. The control group was given only saline. One dose of 20mg/kg MTX intraperitoneal was administered in the MTX group. IB 7mg/kg was given to the MTX+IB (MI) group. Three days after IB was administered, 20mg/kg MTX was given. Five days after MTX was administered, all rats were sacrificed.
ResultsThe TNF-α, ET-1, malondialdehyde (MDA), myeloperoxidase (MPO) and caspase-3 levels in MTX group were significantly higher than in control groups of TNF-α (P=.001), ET-1 (P=.001), MDA (P=.001), MPO (P=.001) and caspase-3 levels (P=.001) and MI groups of TNF-α (P=.009), ET-1 (P=.001), MDA (P=.047), MPO (P=.007) and caspase-3 levels (P=.003). The MI group had less histopathological damage in lung tissue than the MTX group.
ConclusionOverdose of MTX leads to cytokine release and the formation of reactive oxygen species in addition to increased ET-1 secretion release that causes lung damage. IB, as a potent proinflammatory agent, TNF-α blocker, can decrease ET-1 release and oxidative stress, it may show significant protective effects in lung tissue against damage caused by MTX overdose.
El metotrexato (MTX) se emplea para tratar el cáncer, varias formas de artritis y otras patologías reumáticas, pero puede causar toxicidad pulmonar debido a la producción de radicales libres del oxígeno y varias citocinas. Infliximab (IB) es un potente inhibidor del factor de necrosis tumoral-alfa (TNF-α) e inhibe también la liberación de endotelina-1 (ET-1). Nos propusimos investigar si IB reduce el daño pulmonar inducido por una sobredosis de MTX.
MétodoLas ratas se dividieron en 3 grupos de 8 animales. Al grupo control solamente se le administró solución salina. Al grupo MTX se le administró una dosis intraperitoneal de 20mg/kg de MTX. Al grupo de MTX+IB (MI) se le administraron 7mg/kg de IB. Tres días después de la administración de IB se administraron 20mg/kg de MTX. Todas las ratas se sacrificaron 5días después de la administración de MTX.
ResultadosLas concentraciones de TNF-α, ET-1, malondialdehído (MDA), mieloperoxidasa (MPO) y caspasa-3 fueron significativamente más altas en el grupo MTX que en el grupo control: TNF-α (p<0,001), ET-1 (p<0,001), MDA (p<0,001), MPO (p<0,001) y caspasa-3 (p<0,001) y en el grupo MI: TNF-α (p<0,009), ET-1 (p<0,001), MDA (p<0,047), MPO (p<0,007) y caspasa-3 (p<0,003). El grupo MI mostró menos daño histopatológico en el tejido pulmonar que en el grupo MTX.
ConclusiónLa sobredosis de MTX induce la liberación de citocinas y la formación de especies reactivas de oxígeno, además de una mayor secreción de ET-1 que provoca daño pulmonar. IB es un agente proinflamatorio potente, bloquea el TNF-α, puede reducir la liberación de ET-1 y el estrés oxidativo y mostrar importantes efectos protectores del tejido pulmonar frente al daño causado por una sobredosis de MTX.
Methotrexate (MTX) is a folic acid analog widely used to treat systemic inflammatory diseases such as systemic lupus erythematosus, rheumatoid arthritis, psoriasis, as well as many malignancies, including lung and breast cancer.1,2 Pharmacological doses of MTX suppress proinflammatory cytokines, and have a weak tumor necrosis factor-alpha (TNF-α) suppressive effect. Long-term use at therapeutic doses or overdose of MTX can cause significant dosage-dependent pulmonary side effects, such as acute and subacute respiratory failure, nonproductive cough, dyspnea, fever, pneumonitis, interstitial lung disease, and pulmonary fibrosis.2,3
Although the reasons for MTX toxicity in the lungs are unclear, some explanations have been proposed. MTX-induced immune suppression causes recurrent viral or bacterial infections and hypersensitivity reactions. MTX may also have a direct toxic effect on the alveolar epithelial walls.4 Furthermore, MTX causes toxicity by increasing apoptosis and fibrosis of lung tissue.5 Although no human studies have been conducted, experimental studies have shown that MTX may cause acute pulmonary toxicity by increasing secretion of cytokines such as TNF-α, interleukin-1 (IL-1), interleukin-8 (IL-8), and monocyte chemotactic protein-1.6,7 In addition, overdose of MTX can lead to proinflammatory cytokine release due to the increase in oxidative stress and reactive oxygen species (ROS) formation.8 Overdose also leads to pulmonary tissue damage by increasing the caspase enzyme system and activating ROS formation.5,9
Infliximab (Ib), a chimeric monoclonal antibody, is used as an anti-TNF-α agent in rheumatic, gastrointestinal, and dermatological disorders, as well as in chronic eye diseases and sarcoidosis.10–12 Ib targets TNF-α activity in a selection of in vitro bioassays by human fibroblasts, endothelial cells, neutrophils, lymphocytes, and epithelial cells.13 Ib diminishes the secretion of proinflammatory cytokines and reduces the formation of ROS by inhibiting TNF-α. It prevents tissue damage by inhibiting excessive cytokine release and reducing the formation of ROS, and thus reduces tissue damage by decreasing the stimulation of apoptosis.14 In addition to blocking TNF-α and inhibiting endothelin-1 (ET-1), Ib has been reported to have a protective effect in lung tissue. ET-1, a potent vasoconstrictor and bronchoconstrictor, is released from the bronchial epithelium and has been implicated in fibrosis.15 ET-1 increases the release of proinflammatory cytokines, while release of ET-1 increases with increased cytokine levels in lung tissue.16
In this study, we aimed to measure major proinflammatory cytokines ET-1 and TNF-α, malondialdehyde (MDA) levels, and myeloperoxidase (MPO) enzyme activity in lung tissue injury induced by high doses of MTX, in order to evaluate the role of ROS formation and apoptosis. Furthermore, we aimed to investigate whether Ib affects these parameters and has a protective role in lung toxicity caused by MTX overdose.
Materials and MethodsAnimalsThis study was performed on 24 Wistar albino rats. Rats were on average 12–15 weeks old and weighed 250–300g. The experimental animals were randomly divided into 3 groups: a control group (n=8), MTX (n=8) group, and MTX+Ib (MI) group (n=8). The research was conducted according to the Guide for the Care and Use of Laboratory Animals (NIH, 1985) and was approved by local ethics committee (approval number: 2014/12).
Experimental DesignThe control group received isotonic saline solution only (equal to the volume of intraperitoneal MTX). Intraperitoneal injections were performed with 20mg/kg single-dose MTX (Emthexat-s, 50mg ampoule) in the MTX group. In the MI group, the rats were administered a single 7mg/kg Ib dose intraperitoneal injection, and after 3 days, a single 20mg/kg dose of MTX was administered. All rats in all groups were sacrificed on the same day, 5 days after administration of MTX. All groups were anesthetized with ketamine hydrochloride (ketamine and 50mg/kg, intramuscularly, Parke-Davis Eczacibasi, Istanbul, Turkey) before they were sacrificed. Lung tissue from the rats was stored at −80°C until the analysis was conducted.
Tissue HomogenatesLung tissue samples were homogenized with phosphate-buffered saline (PBS; pH 7.4). The samples were centrifuged at 10,000×g for 20min. The supernatant was removed, aliquoted to tubes and frozen at −80°C. The parameters were studied within 1 month.
Measurement of ProteinThe Lowry protocol was used to measure tissue homogenate protein levels. The method is based on the Biuret reaction, in which the peptide bonds of proteins react with copper under alkaline conditions to produce Cu+, which reacts with the Folin reagent (Folin–Ciocalteu reaction).17
Tissue TNF-αTNF-α concentrations were measured using the enzyme-linked immunosorbent assay (ELISA) method. We used the commercially available rat TNF-α ELISA kit (eBioscience, Vienna, Austria).
Tissue MDAThe MDA levels were measured with Draper and Hadley's double-heating method, which is based on spectrophotometric measurement of the color generated by the reaction of thiobarbituric acid (TBA) with MDA. MDA levels are expressed as μmol/L.18
Tissue ET-1ET-1 concentrations were measured using the ELISA method. We used the commercially available rat ET-1 ELISA kit (Elabscience, Wuhan, China).
Tissue MPOMPO concentrations were measured using the ELISA method. We used the commercially available rat MPO ELISA kit (Elabscience, Wuhan, China).
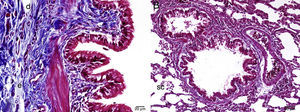
Immunohistological EvaluationFor immunohistochemical staining, 3-μm-thick sections of the lung tissues were cut and placed in xylene for 20min before an alcohol series was implemented (50%–100%). The samples were then placed in an H2O2 solution for 30min. After being washed with PBS, the samples were heated in a citrate buffer solution at 800W for 5min (twice), and placed in a secondary blocker substance for 30min. Each slide stood for 60min in different dilutions of the primary antibody (anti-caspase 3 antibody (ab4051), Abcam, Cambridge, UK). A diaminobenzidine (DAB Substrate Kit, ab64238, Abcam) solution was used as a chromogen, Mayer's hematoxylin as a counterstain for 3–5min, and PBS as a negative control. The preparations were photographed after they had been appropriately covered. After immunohistochemical staining, the preparations were divided into 4 categories according to the tissue percentage of the immunopositive reaction areas: weak (+), moderate (++), strong (+++), and very strong (++++). The blocked tissues were cut into 4-μm-thick sections before being stained with hematoxylin and eosin (H&E) and Masson's trichrome, and then the fields (a total of 150 zones) were photographed for histopathological assessment. The tissues were appraised by blinded grade examination in groups by 2 expert histologists and a pathologist.
Statistical AnalysesStatistical analysis was performed with SPSS for Windows (SPSS, USA) version 17. Continuous variables were reported as mean±standard deviation. Comparison of the groups such as TNF-α, ET-1, MPO, and MDA biochemical parameters analyses were performed using the one-way ANOVA and Bonferroni post hoc tests. The Mann–Whitney U test was used to compare the groups for histopathological parameters. Differences were considered significant at P<.05.
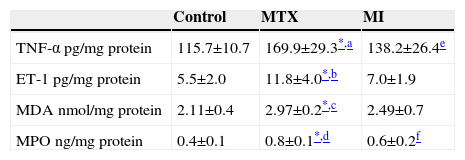
ResultsBiochemical DataThe TNF-α, ET1, MDA, and MPO values of the MTX group were significantly higher than those of the control and MI groups. The TNF-α and MPO values of the MI group were statistically higher than those of the control group. The ET-1 and MDA levels were slightly higher than in the control group, but not statistically significant. All biochemical results are shown in Table 1.
Biochemical Result From All Three Groups.
| Control | MTX | MI | |
|---|---|---|---|
| TNF-α pg/mg protein | 115.7±10.7 | 169.9±29.3*,a | 138.2±26.4e |
| ET-1 pg/mg protein | 5.5±2.0 | 11.8±4.0*,b | 7.0±1.9 |
| MDA nmol/mg protein | 2.11±0.4 | 2.97±0.2*,c | 2.49±0.7 |
| MPO ng/mg protein | 0.4±0.1 | 0.8±0.1*,d | 0.6±0.2f |
Abbreviations: Mtx: methotrexate; MI: methotrexate+infliximab; TNF-α: tumor necrosis factor-alpha; ET-1: Endothelin-1; MDA: malondialdehyde; MPO: myeloperoxidase.
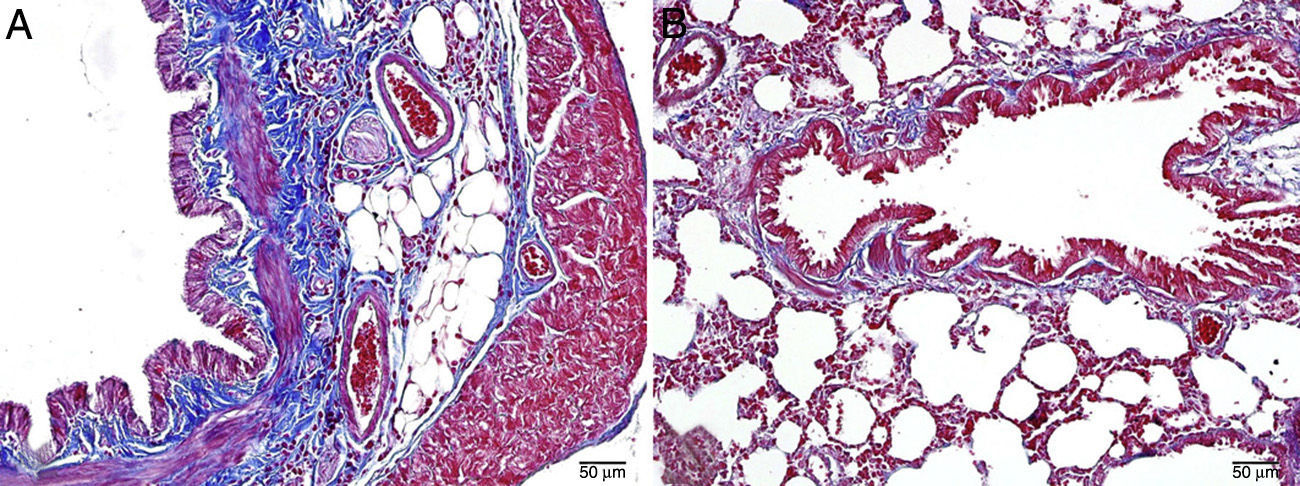
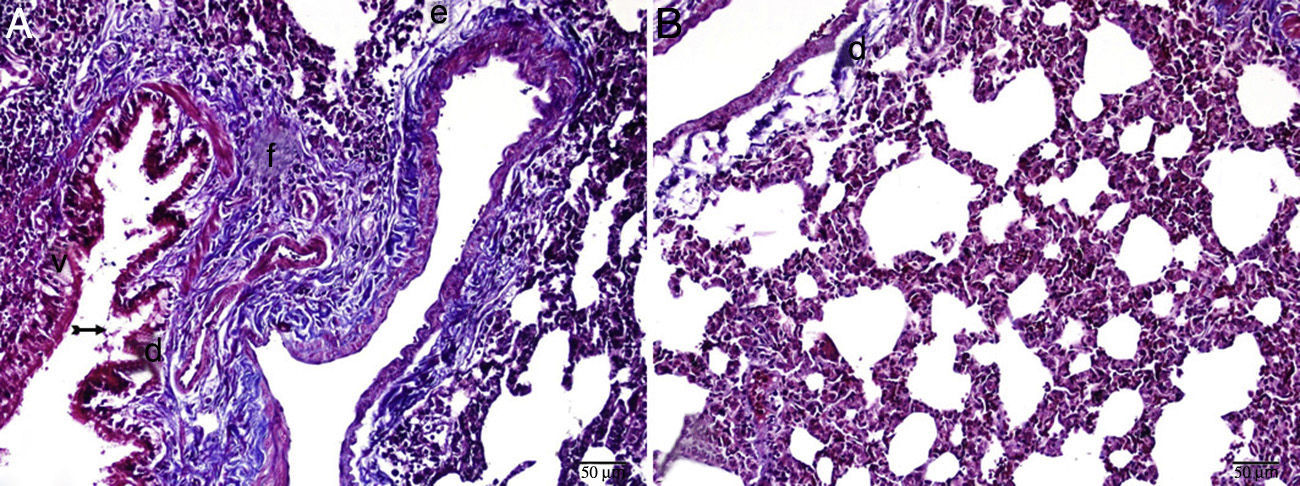
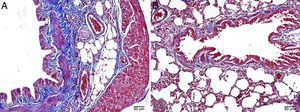
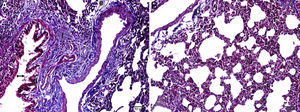
The histopathological study in the control group indicated that tissue was healthy and histological morphology was normal. Although the range of interstitial inflammation in lung tissue with normal histological appearance and congestion score was zero, connective tissue and vascular structures were also normal despite a slight loss in epithelial cells (Fig. 1).
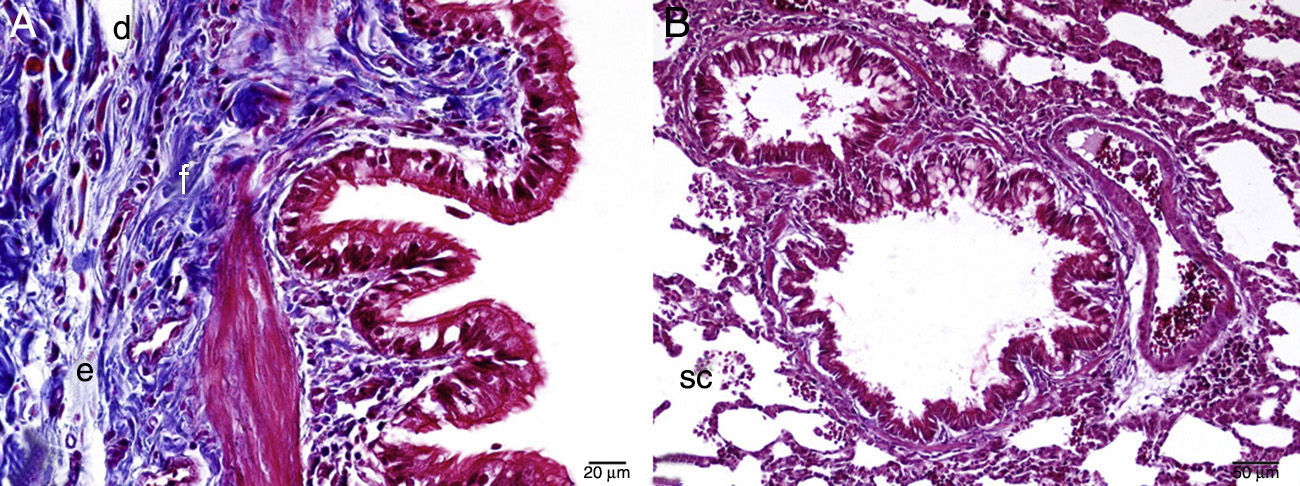
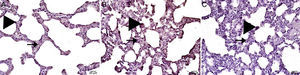
Interstitial edema, alveolar epithelial injury, polymorphonuclear leukocyte infiltration, and chronic inflammatory cell infiltrates were observed in the rats treated with MTX alone (Fig. 2). In the lung tissue of the MTX group, which did not have a normal histological appearance, interstitial inflammation, and congestion scores of 2+, 3+, and 4+ were observed. Although the inflammation score was more intense in the 2–3 range, the proportion of congestion was more intense in the 3+ to 4+ range.
In the MI group, the congestion decreased compared to the MTX group. Although there was little change in the fibrosis intensity, epithelial loss and polymorphonuclear leukocyte infiltration had decreased. A significant decrease in alveolar epithelial damage, edema, polymorphonuclear leukocyte infiltration, and chronic inflammatory cell infiltration was observed in the rats pretreated with Ib. Less interstitial edema, alveolar epithelial injury, polymorphonuclear leukocyte infiltration, and chronic inflammatory cell infiltration were observed in the lungs of animals in the group treated with Ib (P<.05) (Fig. 3).
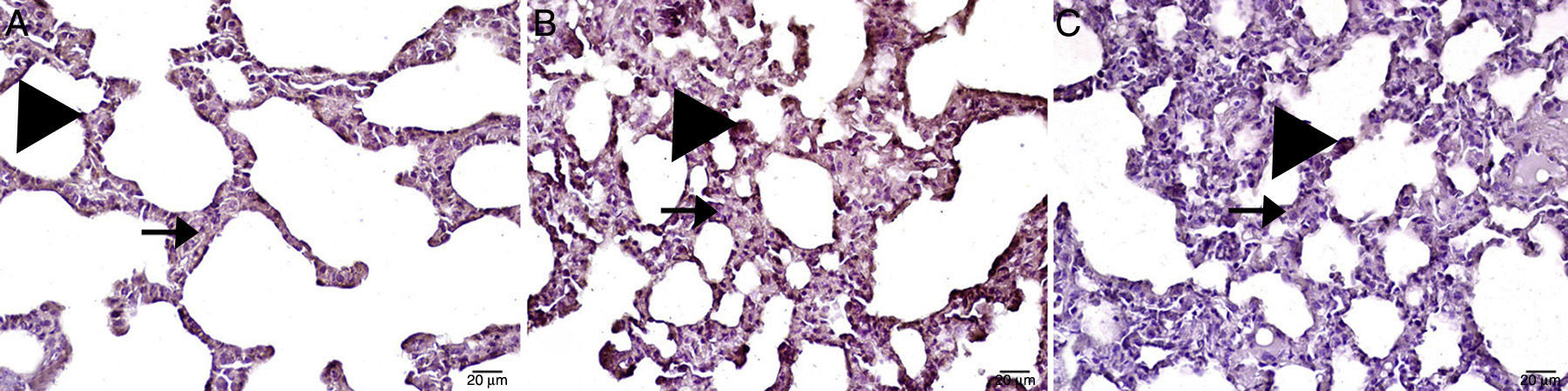
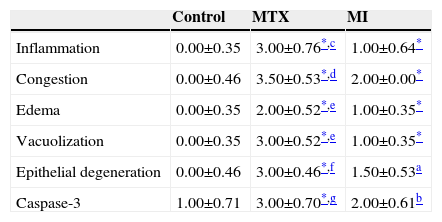
Caspase-3Caspase-3 levels in the MTX group were significantly higher than in the control group and the MI group. Caspase-3 in the MI group was significantly higher than the control group, but was much lower than in the MTX group. All histopathological results are shown in Table 2 and Fig. 4.
Histopathologic Examination of Lung Tissue.
| Control | MTX | MI | |
|---|---|---|---|
| Inflammation | 0.00±0.35 | 3.00±0.76*,c | 1.00±0.64* |
| Congestion | 0.00±0.46 | 3.50±0.53*,d | 2.00±0.00* |
| Edema | 0.00±0.35 | 2.00±0.52*,e | 1.00±0.35* |
| Vacuolization | 0.00±0.35 | 3.00±0.52*,e | 1.00±0.35* |
| Epithelial degeneration | 0.00±0.46 | 3.00±0.46*,f | 1.50±0.53a |
| Caspase-3 | 1.00±0.71 | 3.00±0.70*,g | 2.00±0.61b |
Abbreviations: MTX, methotrexate; MI, methotrexate+infliximab.
Our study shows that TNF-α (a proinflammatory cytokine), MDA (a good marker of oxidative stress), MPO (a component of the antioxidant defense system), and ET-1 (one of the most potent vasoconstrictors) were significantly higher in the MTX-induced lung toxicity group than in the other two groups. In the histopathological study with H&E compared to the other two groups, histological damage was found in lung tissue. Caspase-3, a good indicator of apoptosis, was significantly higher in the MTX group compared to the other groups. However, the TNF-α, MDA, MPO, and ET-1 levels in the MI group were significantly lower than these levels in the MTX group. ET-1 and MDA levels in the MI group were slightly higher than those of the control group; however, TNF-α and MPO levels were significantly higher in the MI group than in the control group. According to the histopathological study, the MI group showed less histological damage than the MTX group. Furthermore, caspase-3 levels were significantly lower in the MI group than in the MTX group.
MTX inhibits tissue macrophage infiltration, and thus affects the synthesis of interleukins. However, MTX does not affect tissue infiltration of T-lymphocytes. Since TNF-α is expressed on T-lymphocytes, MTX cannot inhibit TNF-α levels.19 MTX may cause pneumonitis by increasing the secretion of IL-8 in the airway epithelium.20 Toxic doses of MTX and interleukin-1 beta (IL-1β) increase the secretion of proinflammatory cytokines such as TNF-α and MDA.21–23 IL-1β and TNF-α secretion increase pulmonary toxicity by inducing IL-8 secretion.6 TNF-α is one of the most crucial substrates responsible for the pathogenesis of chronic inflammatory diseases and oxidative stress. TNF-α has multifunctional features, including regulating the growth, proliferation, differentiation, and viability of activated leukocytes. TNF-α also provokes the cellular release of other cytokines, chemokines, or inflammatory mediators.24 Exclusively, TNF-α inhibition plays a pivotal role in the recruitment cascade of many rheumatic diseases by inhibiting the inflammation process. Since TNF-α not only increases ROS but also activates the caspase enzyme system, excessive apoptosis is induced, leading to tissue damage. In this study, therefore, we considered that the evaluation and analysis of TNF-α levels would suffice to assess cytokine status. Studies have shown that Ib inhibits IL-1β and TNF-α.25 In another study, Ib was reported to protect against lipid peroxidation damage and dense tissue cytokine release by suppressing TNF-α and reducing ROS and MDA.26 In this study, TNF-α levels in the group treated with MTX were significantly higher, and the rats had significant tissue damage. Increased cytokine release due to increasing apoptosis (excessive release of cytokines and enhanced ROS) and increasing IL-8 release might have damaged the lung tissue. In the MI group, TNF-α was suppressed, and there was less tissue damage. Ib in the MI group might have prevented lung damage by blocking TNF-α and lipid peroxidation and reducing apoptosis. Corrado et al.27 reported that MTX combined with Ib further suppressed cytokines such as interleukin-6 (IL-6) and prevented the negative effects of MTX on bone metabolism, and would be a good combination if used together. In this study, the negative effects of overdose of MTX on cytokine balance were reduced by the use of Ib.
Lipid peroxidation leads to cell and tissue damage, creating highly toxic ROS, either via a direct toxic effect or by inducing apoptosis. Oxidative stress also damages the lungs and causes pulmonary fibrosis.28 Although MTX causes acute pulmonary pneumonitis, MTX can also produce chronic pulmonary fibrosis.1 Overdose of MTX leads to excessive cytokine release and the formation of ROS, further increasing oxidative stress damage by causing weakness in the antioxidant defense system. Ib reduces oxidative stress and strengthens the antioxidant defense system. MDA, the end product of the lipid peroxidation process, is often used for measuring oxidative damage to lipids resulting from free radicals.29 Therefore, we considered that analysis of MDA, a good marker of oxidative stress, was sufficient for the assessment of oxidative stress, which in this study was significantly higher in the MTX group. In the Ib group, MDA was significantly lower than in the MTX group. According to the results of this study, while MTX damages lung tissue by increasing oxidative stress, Ib counters this damage by reducing oxidative stress.
MPO, secreted from monocytes and neutrophils and activated in response to increased oxidative stress, is a heme-containing enzyme. It disrupts the structure of the protein by using nitric oxide as a substrate and leads to endothelial dysfunction. This enzyme leads to tissue damage by activating matrix metalloproteinase via the substrate it forms. Furthermore, increased MPO is a direct indicator of oxidative stress.30 In this regard, its levels have been considered an excellent determinant for oxidative stress. MPO activity increases in MTX toxicity,22 while increased MPO has been implicated in lung tissue damage.31 Ib suppresses MPO enzyme activity, thus reducing oxidative stress and lung tissue damage.26,32 This study showed that although MPO levels were high in the MTX group, its activity was regulated in the Ib group. Overdose of MTX damages lung tissue by increasing oxidative stress and MPO activity, but Ib might have prevented this damage by decreasing both.
ET-1, produced in smooth muscle cells, the airway epithelium, and alveolar epithelial cells, contributes significantly to the development of pulmonary fibrosis.33 Furthermore, it causes pulmonary damage by increasing shear stress, stimulating the secretion of thrombin, angiotensin II, and cytokines, and boosting free radical formation.34 MTX is known to damage endothelial cells,6 but no studies on its direct effect on ET-1 have been published. In our study, significant ET-1 levels were found in the MTX group. This drug may cause dysfunction and endothelial damage via excessive cytokine release and ROS formation, which may lead to increased ET-1 secretion in the endothelium. However, ET-1 secretion may also increase pulmonary damage due to increased cytokine release and ROS formation. On one hand, ET-1 inhibits apoptosis dose-dependently and has a mitogenic effect.16 However, if mitochondrial dysfunction exists, ET-1 secretion and gene expression increase, and ET-1 overproduction induces apoptosis by increasing caspase-3.35 This study showed that increased cytokine release and ROS formation can lead to increased ET-1 gene expression that may cause ET-1 overproduction, stimulated by caspase-3 enzyme activity. As a result, pulmonary damage occurs due to induced apoptosis. Additionally, TNF-α escalates the release of ET-1.36 TNF-α inhibition with Ib alleviates asthma37 and reduces ET-1 levels,38 so in this study, the TNF-α inhibitory action of Ib might have reduced ET-1 levels and lung damage.
ROS damage in the lung tissue produces cell swelling, alveolar edema, and congestion, resulting in acute inflammation, with edema and neutrophil migration. Neutrophils cause ROS formation and tissue damage by releasing a chemical mediator. Lipid peroxidation results in organ and cell damage. TNF-α is an effective pleiotropic cytokine that mediates inflammation and cellular immune responses, affecting not only the growth but also the differentiation and function of many cell types, thus ensuring the destruction of damaged cells by apoptosis. However, excessive TNF-α secretion leads to tissue damage by inducing oxidative stress and directly inducing apoptosis.
There are few specific, reliable means of detecting of apoptosis. Nonetheless, caspase-3 signals the induction of cell death, a key element in this process. Caspase-3 activation often leads to the irreversible commitment of a cell to apoptosis. Thus, caspase-3 has been reported to be a valuable marker of apoptosis.39 In this study, we evaluated caspase-3 activity in order to determine apoptotic status. We found that caspase-3 and histological damage was much higher in the MTX group than in the treatment group. MTX, associated with excessive cytokine levels, ET-1 secretion and ROS formation, may have caused lung damage by activating the caspase-3 pathway. Ib is known to regulate apoptosis,40 and indeed, caspase-3 activation and histological damage were lower in the Ib treatment group. According to the results of this study, TNF-α blockade produced by Ib may have prevented the excessive cytokine secretion induced by the MTX overdose, and thus may have reduced ET-1 secretion and ROS formation. Therefore, Ib may have prevented lung tissue damage by preventing caspase-3 activation.
ConclusionOverdose of MTX with intense cytokine release and ROS formation causes acute lung damage. Additionally, MTX might have resulted in increased ET-1 secretion, and excess ET-1 levels might have contributed to tissue damage. TNF-α blockade with Ib may have protected the lung tissue by reducing ET-1 secretion and ROS formation and inhibiting induction of apoptosis. Ib and MTX may be a good combination, since Ib reduces the toxic effects of MTX.
Conflict of InterestThe authors declare no conflict of interest.
Please cite this article as: Kurt A, Tumkaya L, Turut H, Cure MC, Cure E, Kalkan Y, et al. Efectos protectores de infliximab sobre el daño pulmonar inducido por metotrexato. Arch Bronconeumol. 2015;51:551–557.