To evaluate the effect of continuous positive airway pressure (CPAP) on the nostrils of patients with sleep apnea–hypopnea syndrome and its impact on quality of life, and to identify predictive factors for compliance.
MethodsLongitudinal prospective study. Thirty-six consecutive patients evaluated before and 2 months after CPAP using the following variables: clinical (eye, nose and throat [ENT] symptoms, Epworth test, anxiety/depression scales, general and rhinoconjunctivitis-specific quality of life); anatomical (ENT examination, computed tomography); functional (auditive and Eustachian tube function, nasal flow, mucociliary transport); biological (nasal cytology); and polisomnographics. The sample was divided into partients with good adherence (≥4h/d) and patients with bad adherence (<4h/d).
ResultsA significant improvement was observed in daytime sleepiness (P=.000), anxiety (P=.006), and depression (P=.023). Nasal dryness (P=.000), increased neutrophils in nasal cytology (P=.000), and deteriorating ciliary function were evidenced, particularly in partients with good adherence. No significant differences were observed in the other variables. Baseline sleepiness was the only factor predictive of compliance.
ConclusionsCPAP in patients without previous nasal pathology leads to an improvement in a series of clinical parameters and causes rhinitis and airway dryness. Some ENT variables worsened in partients with good adherence. Sleepiness was the only prognostic factor for poor tolerance.
Evaluar el efecto de la presión positiva continua en la vía aérea (CPAP) sobre las fosas nasales de pacientes con síndrome de apnea hipopnea del sueño y su impacto en la calidad de vida, e identificar factores predictivos de cumplimiento.
MétodosEstudio prospectivo longitudinal. Treinta y seis pacientes consecutivos evaluados antes y 2 meses tras CPAP usando las siguientes variables clínicas (síntomas otorrinolaringológicos, test de Epworth, escala de ansiedad/depresión, calidad de vida general y específica para rinoconjuntivitis); anatómicas (exploración otorrinolaringológica, tomografía computarizada); funcionales (función auditiva y tubárica, flujo nasal, transporte mucociliar); biológicas (citología nasal), y polisomnográficas. Se dividió la muestra entre cumplidores (≥4h/d) y no cumplidores (<4h/d).
ResultadosSe objetivó una mejoría significativa en la somnolencia diurna (p=0,000), la ansiedad (p=0,006) y la depresión (p=0,023). Se evidenció sequedad nasal (p=0,000), aumento de neutrófilos en la citología nasal (p=0,000) y deterioro de la función ciliar, especialmente en cumplidores. No se evidenciaron diferencias significativas en el resto de las variables. La somnolencia inicial fue el único factor pronóstico de cumplimiento.
ConclusionesEl tratamiento con CPAP en pacientes sin patología nasal previa mejora una serie de parámetros clínicos y provoca rinitis y sequedad en la vía aérea. Algunas de las variables otorrinolaringológicas empeoran en los cumplidores. La somnolencia fue el único factor pronóstico de mala tolerancia.
Sleep apnea–hypopnea syndrome (SAHS) is characterized by complete or partial obstruction of the upper airway while the patient is asleep, accompanied by oxyhemoglobin desaturation or micro-awakenings. SAHS is associated with a higher risk of cardiovascular disease, arterial hypertension, neurocognitive changes and death.1 Continuous positive airway pressure (CPAP) is the treatment of choice in most patients with moderate-severe SAHS, and helps control the disease, improves quality of life and reduces morbidity and mortality.2
Treatment must be applied continuously to be effective, but adverse reactions mean that mid-term compliance is only achieved in 60% of patients.3 CPAP tolerability and compliance studies have shown varying results.4 Lack of compliance has been associated with mask discomfort, feeling of claustrophobia, noise disturbance, nasal inflammation, pharyngeal dryness, eye symptoms, non-specific ear discomfort, sleepiness or excessive fatigue.5,6 The patient's psychological profile must also be taken into account, and educational and behavioral support play an important role in improving tolerability and compliance.7,8
CPAP-related rhinitis is a significant problem, but it is not always associated with poorer compliance.9 We hypothesized that an in-depth study of changes occurring in the eyes, nose and throat (ENT) of patients receiving CPAP treatment could lead to the detection and early treatment of potential patients with bad adherence, and that personalized medicine in these cases will optimize management.
Our objective was to analyze changes occurring in the nostrils of SAHS patients with no previous nasal pathology after the introduction of CPAP, and the impact on treatment compliance.
Materials and MethodsDesignThis was a prospective longitudinal study. Forty-one CPAP candidates, consecutively diagnosed with SAHS on polysomnography (apnea–hypopnea index [AHI]>10), were evaluated before and 2 months after starting CPAP treatment according to clinical, anatomical, functional, biological and polysomnographic variables. Each of the ENT evaluations and additional tests was performed by the same assessor, depending on the variable, before and after CPAP treatment. In all cases, the assessor was blinded as to the results of the other variables.
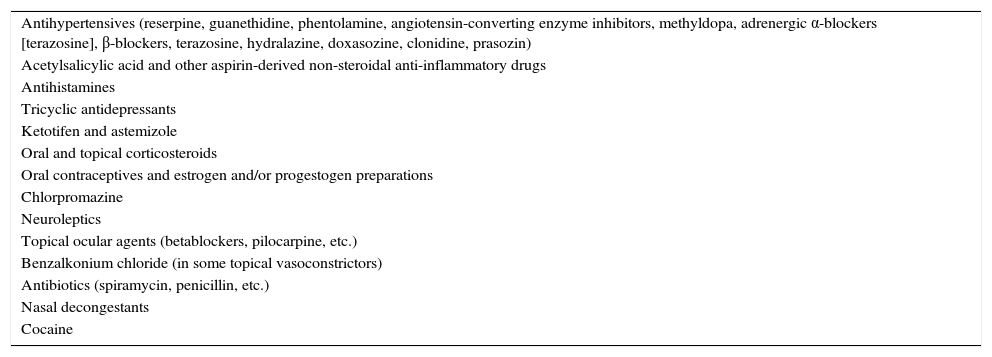
Inclusion and Exclusion CriteriaOnly patients over 18 years of age were included. Excluded patients were those taking corticosteroids, antihistamines (nasal or systemic), or rhinitis-inducing medications (Table 1); patients previously treated with CPAP, BIPAP or oxygen; and patients with central apneas or previous nasal pathologies. All patients signed informed consent before study start.
Rhinitis-inducing Drugs.
| Antihypertensives (reserpine, guanethidine, phentolamine, angiotensin-converting enzyme inhibitors, methyldopa, adrenergic α-blockers [terazosine], β-blockers, terazosine, hydralazine, doxasozine, clonidine, prasozin) |
| Acetylsalicylic acid and other aspirin-derived non-steroidal anti-inflammatory drugs |
| Antihistamines |
| Tricyclic antidepressants |
| Ketotifen and astemizole |
| Oral and topical corticosteroids |
| Oral contraceptives and estrogen and/or progestogen preparations |
| Chlorpromazine |
| Neuroleptics |
| Topical ocular agents (betablockers, pilocarpine, etc.) |
| Benzalkonium chloride (in some topical vasoconstrictors) |
| Antibiotics (spiramycin, penicillin, etc.) |
| Nasal decongestants |
| Cocaine |
Daytime sleepiness was quantified according to the Epworth scale,10 with a score of >11 being considered excessive.11 Patient mood was determined using the hospital anxiety-depression scale (HAD)12and general quality of life was assessed using the SF-12 questionnaire.13
ENT clinical symptoms were studied as follows: Nasal congestion was evaluated according to the Nasal Obstruction Symptom Evaluation scale (NOSE14; 0=no obstruction, 1=mild obstruction, 2=moderate obstruction, 3=severe obstruction). Rhinitis symptoms (nasal secretion, nasal pruritus, and sneezing) were classified according to the Rasp staging system15 (0=asymptomatic, 1=mild symptoms, 2=moderate symptoms, 3=severe symptoms). Nasopharyngeal dryness, hearing problems (hypoacusia and tinnitus) and skin and eye problems (skin discomfort in the area of contact with the mask and eye irritation from mask air leaks) were evaluated individually on a scale of 0–3, in a similar manner to the Rasp scale. Specific quality of life associated with ENT symptoms was studied using the Rhinoconjunctivitis Quality of Life Questionnaire.16
Anatomical VariablesOtoscopy and rhinoscopy were classified as normal or abnormal.
Excess weight or obesity was evaluated using the body mass index and waist circumference.
Nasal cavity volumes were calculated in cm3 from multidetector computed tomography (CT) images (Siemens Sensation 64 with 3mm slices).
Functional VariablesNasal respiratory flow (cm3/s) in each nostril was evaluated by active anterior rhinomanometry (Rinospir Pro Rhinometer, SIBEL S.A., Spain) at a pressure of 150Pa with the patient in a sitting position. Total flow (right+left) was considered normal at >630cm3/s in women and >700cm3/s in men.17
Mucociliary clearance was measured using the saccharin test,18 with a score of >60minutes considered abnormal.
Tubaric function19 was evaluated by tympanometry (GSI TympStar impedance meter, Grason-Stadler, Eden Prairie, USA). Maximal distensibility between +50 and −100mmH2O was considered normal. Auditive function19 was evaluated by pure-tone audiometry (GSI TympStar audiometer, Grason-Stadler, Eden Prairie, USA) at frequencies of 250–8000Hz. Normal hearing was response to a stimulus of <25 decibels at conversational frequencies.
Biological VariablesTotal immunoglobulin E in blood (normal≤100U/ml (240mg/l) and eosinophils in blood (normal<5%) were determined. The presence of nasal inflammation was evaluated by cytology obtained from curettage of the inferior turbinate mucosa (Rhinoprobe®). The number of eosinophils and neutrophils was calculated on a basis of 500 epithelial cells (neither eosinophils nor neutrophils are observed in healthy, disease-free individuals).
Polysomnography VariablesRespiratory variables were evaluated according to Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) guidelines,20,21 and neurological variables according to the guidelines of Rechtschaffen and Kales.22AHI, percentage of time with oxygen saturation <90% (CT 90), maximum oxygen desaturation during the night (nadir) and CPAP pressure were quantified.
Follow-upAll patients were monitored in the hospital's Sleep Unit following standard practices, which include an initial visit at 2–4 weeks for CPAP titration. Patients with no specific difficulties were monitored 2–3 months later, and those who required specific readjustments were monitored as needed, until the problem was solved. The CPAP device, equipped to detect residual events, CPAP pressure, leaks and compliance over a period of several days, was used to monitor for intolerance and non-compliance at the recommended pressure.
In line with our working protocol, all study variables were re-evaluated 2 months after starting CPAP, during which time none of the patients received treatment which might have altered the results.
Compliance was evaluated subjectively using a standardized questionnaire and a visual analog scale (0–10), and automatically from the CPAP counter recordings (time in hours and night of CPAP use). Adequate compliance was considered as 4hours/day every day of the week23; according to these results, 2 study groups were established, partients with good adherence (≥4h/d) and patients with bad adherence (<4h/d).
Statistical MethodologyVariables were collected in a database and analyzed using the SPSS 18 statistical package. Quantitative variables are expressed as mean±standard deviation or as percentages, as applicable. The Wilcoxon test or the Chi-squared test was used to analyze variables before and after CPAP. The Mann–Whitney U test was used to compare differences between partients with good adherence and patients with bad adherence. A multivariate analysis was performed to evaluate predictive factors for lack of CPAP compliance. A P-value of <.05 was considered statistically significant.
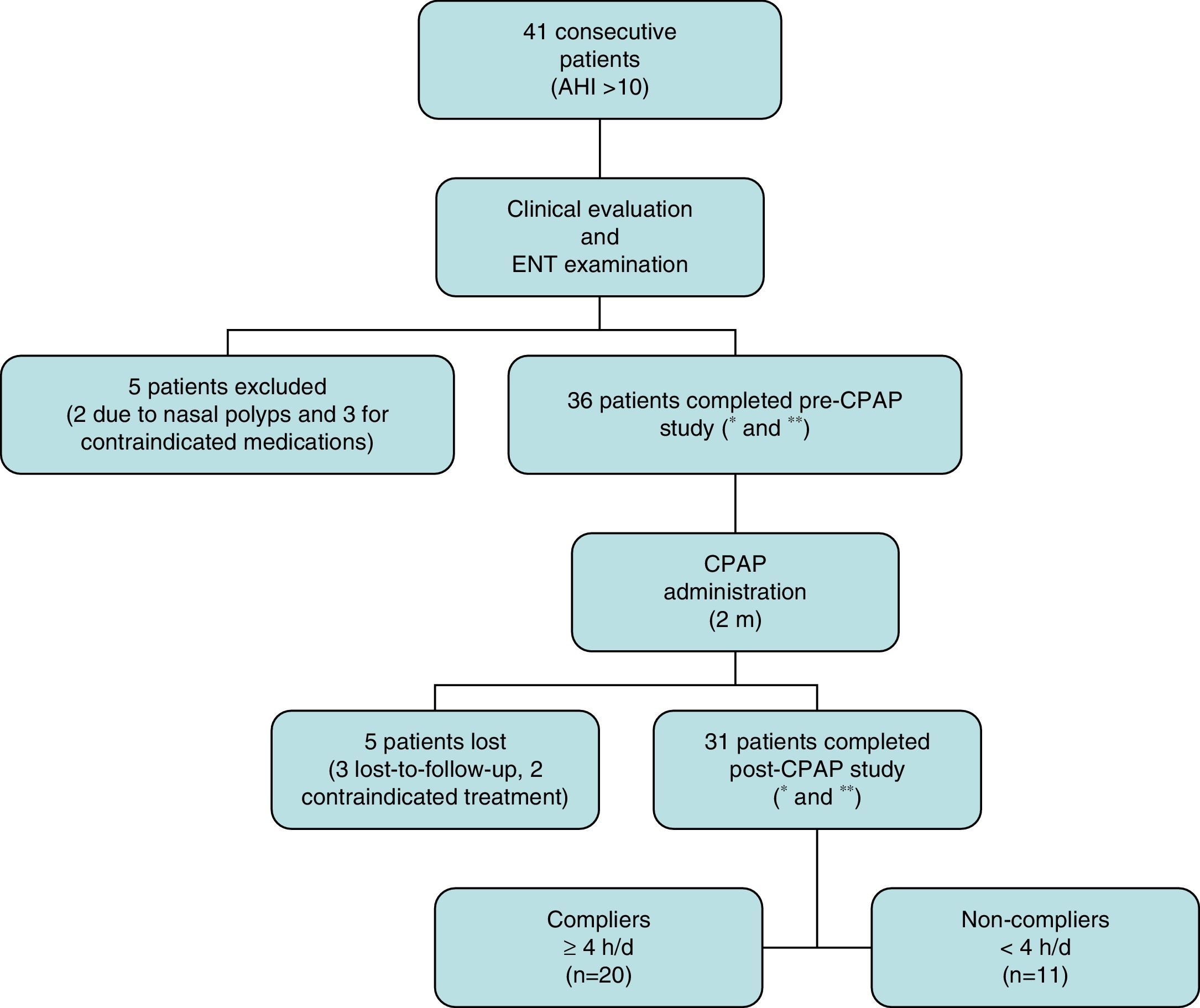
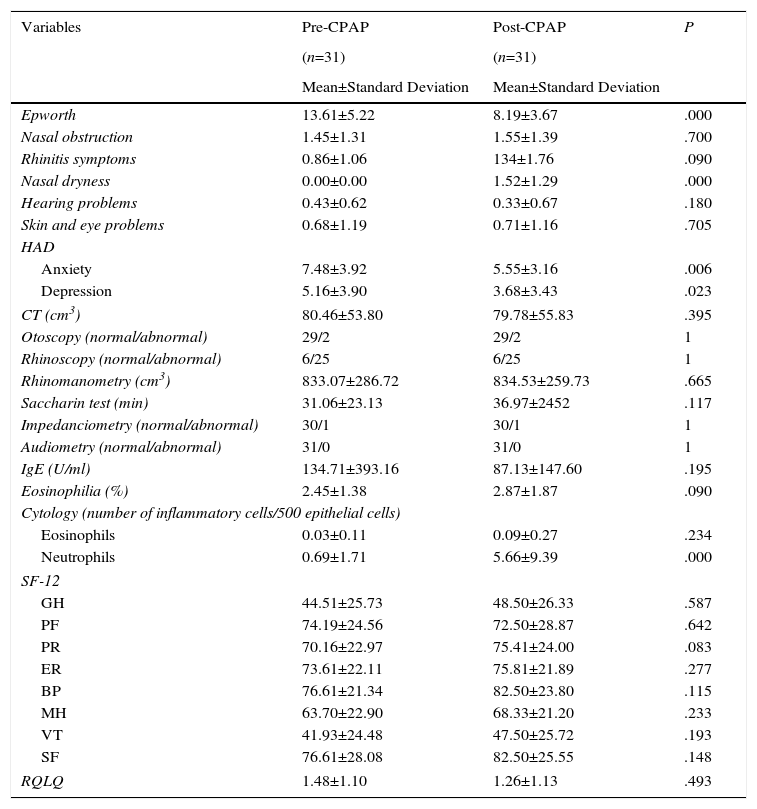
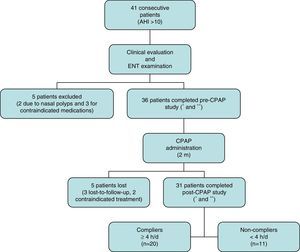
ResultsA total of 41 consecutive CPAP candidates with a diagnosis of SAHS were included. Five were excluded after the initial evaluation when exclusion criteria were detected. Of the remaining 36 (31 men and 5 women with a mean age of 55.72±9.50 years), only 31 completed the 2-month study (Fig. 1). Twenty of these were partients with good adherence and 11 were patients with bad adherence.Results of variables before and after CPAP are described in Table 2. A significant improvement in daytime sleepiness (P=.000), anxiety (P=.006), and depression (P=.023) was observed. Nasal dryness (P=.000) and elevated neutrophils in nasal cytology (P=.000) were also detected. No significant differences were found among the other variables.
Patient disposition.
*Includes full medical history, body mass index, head circumference, otoscopy and flexible fiberoptic nasal endoscopy.
**Includes standardized symptom and quality of life questionnaires, audiometry, impedanciometry, rhinomanometry, saccharin test, nasal curettage, and nasal cavity computed tomography.
AHI: apnea–hypopnea index; CPAP: continuous positive airway pressure; ENT: eyes, nose and throat; h/d: hours/day; m: months.
Clinical, Anatomical, Functional, Biological and Quality of Life Variables Before and After CPAP in all Study Patients.
| Variables | Pre-CPAP | Post-CPAP | P |
|---|---|---|---|
| (n=31) | (n=31) | ||
| Mean±Standard Deviation | Mean±Standard Deviation | ||
| Epworth | 13.61±5.22 | 8.19±3.67 | .000 |
| Nasal obstruction | 1.45±1.31 | 1.55±1.39 | .700 |
| Rhinitis symptoms | 0.86±1.06 | 134±1.76 | .090 |
| Nasal dryness | 0.00±0.00 | 1.52±1.29 | .000 |
| Hearing problems | 0.43±0.62 | 0.33±0.67 | .180 |
| Skin and eye problems | 0.68±1.19 | 0.71±1.16 | .705 |
| HAD | |||
| Anxiety | 7.48±3.92 | 5.55±3.16 | .006 |
| Depression | 5.16±3.90 | 3.68±3.43 | .023 |
| CT (cm3) | 80.46±53.80 | 79.78±55.83 | .395 |
| Otoscopy (normal/abnormal) | 29/2 | 29/2 | 1 |
| Rhinoscopy (normal/abnormal) | 6/25 | 6/25 | 1 |
| Rhinomanometry (cm3) | 833.07±286.72 | 834.53±259.73 | .665 |
| Saccharin test (min) | 31.06±23.13 | 36.97±2452 | .117 |
| Impedanciometry (normal/abnormal) | 30/1 | 30/1 | 1 |
| Audiometry (normal/abnormal) | 31/0 | 31/0 | 1 |
| IgE (U/ml) | 134.71±393.16 | 87.13±147.60 | .195 |
| Eosinophilia (%) | 2.45±1.38 | 2.87±1.87 | .090 |
| Cytology (number of inflammatory cells/500 epithelial cells) | |||
| Eosinophils | 0.03±0.11 | 0.09±0.27 | .234 |
| Neutrophils | 0.69±1.71 | 5.66±9.39 | .000 |
| SF-12 | |||
| GH | 44.51±25.73 | 48.50±26.33 | .587 |
| PF | 74.19±24.56 | 72.50±28.87 | .642 |
| PR | 70.16±22.97 | 75.41±24.00 | .083 |
| ER | 73.61±22.11 | 75.81±21.89 | .277 |
| BP | 76.61±21.34 | 82.50±23.80 | .115 |
| MH | 63.70±22.90 | 68.33±21.20 | .233 |
| VT | 41.93±24.48 | 47.50±25.72 | .193 |
| SF | 76.61±28.08 | 82.50±25.55 | .148 |
| RQLQ | 1.48±1.10 | 1.26±1.13 | .493 |
BP: body pain; CT: computed tomography; GH: general health; HAD: Hospital Anxiety and Depression scale; IgE: immunoglobulin E; MH: mental health; PF: physical function; ER: emotional role; PR: physical role; RQLQ: Rhinoconjunctivitis Quality of Life Questionnaire; SF: social function; SF-12: abbreviated SF-36 quality of life questionnaire; VT: vitality.
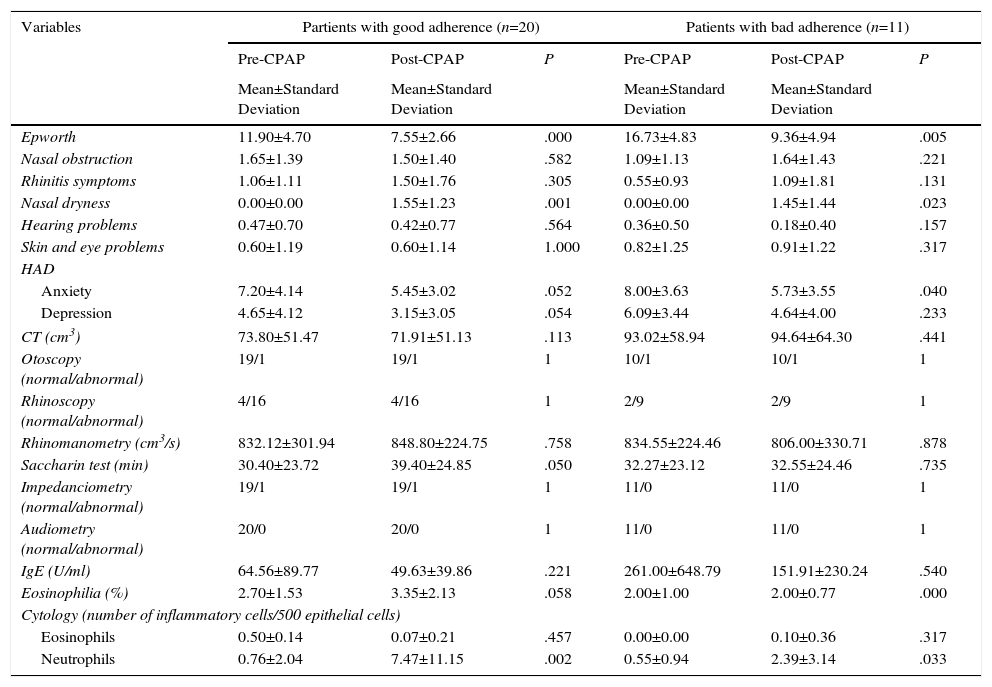
Partients with good adherence (Table 3) showed a more significant improvement on the Epworth test (P=.000), and anxiety (P=.052) and depression (P=.054) showed a clear trend toward improvement. Surprisingly, partients with good adherence had a higher rate of nasal dryness (P=.001), higher neutrophil levels in nasal cytology (P=.002), tended to have worsening mucociliary clearance (P=.050), and blood eosinophils were higher (P=.058) but within normal limits (<5%).
Clinical, Anatomical, Functional, Biological and Quality of Life Variables Before and After CPAP in Partients with good adherence and Patients with bad adherence.
| Variables | Partients with good adherence (n=20) | Patients with bad adherence (n=11) | ||||
|---|---|---|---|---|---|---|
| Pre-CPAP | Post-CPAP | P | Pre-CPAP | Post-CPAP | P | |
| Mean±Standard Deviation | Mean±Standard Deviation | Mean±Standard Deviation | Mean±Standard Deviation | |||
| Epworth | 11.90±4.70 | 7.55±2.66 | .000 | 16.73±4.83 | 9.36±4.94 | .005 |
| Nasal obstruction | 1.65±1.39 | 1.50±1.40 | .582 | 1.09±1.13 | 1.64±1.43 | .221 |
| Rhinitis symptoms | 1.06±1.11 | 1.50±1.76 | .305 | 0.55±0.93 | 1.09±1.81 | .131 |
| Nasal dryness | 0.00±0.00 | 1.55±1.23 | .001 | 0.00±0.00 | 1.45±1.44 | .023 |
| Hearing problems | 0.47±0.70 | 0.42±0.77 | .564 | 0.36±0.50 | 0.18±0.40 | .157 |
| Skin and eye problems | 0.60±1.19 | 0.60±1.14 | 1.000 | 0.82±1.25 | 0.91±1.22 | .317 |
| HAD | ||||||
| Anxiety | 7.20±4.14 | 5.45±3.02 | .052 | 8.00±3.63 | 5.73±3.55 | .040 |
| Depression | 4.65±4.12 | 3.15±3.05 | .054 | 6.09±3.44 | 4.64±4.00 | .233 |
| CT (cm3) | 73.80±51.47 | 71.91±51.13 | .113 | 93.02±58.94 | 94.64±64.30 | .441 |
| Otoscopy (normal/abnormal) | 19/1 | 19/1 | 1 | 10/1 | 10/1 | 1 |
| Rhinoscopy (normal/abnormal) | 4/16 | 4/16 | 1 | 2/9 | 2/9 | 1 |
| Rhinomanometry (cm3/s) | 832.12±301.94 | 848.80±224.75 | .758 | 834.55±224.46 | 806.00±330.71 | .878 |
| Saccharin test (min) | 30.40±23.72 | 39.40±24.85 | .050 | 32.27±23.12 | 32.55±24.46 | .735 |
| Impedanciometry (normal/abnormal) | 19/1 | 19/1 | 1 | 11/0 | 11/0 | 1 |
| Audiometry (normal/abnormal) | 20/0 | 20/0 | 1 | 11/0 | 11/0 | 1 |
| IgE (U/ml) | 64.56±89.77 | 49.63±39.86 | .221 | 261.00±648.79 | 151.91±230.24 | .540 |
| Eosinophilia (%) | 2.70±1.53 | 3.35±2.13 | .058 | 2.00±1.00 | 2.00±0.77 | .000 |
| Cytology (number of inflammatory cells/500 epithelial cells) | ||||||
| Eosinophils | 0.50±0.14 | 0.07±0.21 | .457 | 0.00±0.00 | 0.10±0.36 | .317 |
| Neutrophils | 0.76±2.04 | 7.47±11.15 | .002 | 0.55±0.94 | 2.39±3.14 | .033 |
| Variables | Partients with good adherence (n=20) | Patients with bad adherence (n=11) | ||||
|---|---|---|---|---|---|---|
| Pre-CPAP | Post-CPAP | P | Pre-RECPAP | Post-CPAP | P | |
| Mean±Standard Deviation | Mean±Standard Deviation | Mean±Standard Deviation | Mean±Standard Deviation | |||
| SF-12 | ||||||
| GH | 50.71±23.47 | 48.50±26.06 | .491 | 31.50±26.56 | 48.50±28.28 | .213 |
| PF | 73.81±25.59 | 73.75±26.25 | .917 | 75.00±23.57 | 70.00±34.96 | .414 |
| PR | 72.62±22.57 | 80.00±23.79 | .079 | 65.00±24.15 | 66.25±22.85 | .705 |
| ER | 73.21±22.11 | 77.50±20.92 | .415 | 67.50±24.43 | 71.25±24.33 | .524 |
| BP | 79.76±20.34 | 83.75±24.70 | .454 | 70.00±22.97 | 80.00±22.97 | .046 |
| MH | 66.67±20.66 | 70.00±20.03 | .483 | 57.50±27.13 | 65.00±24.15 | .304 |
| VT | 41.67±24.15 | 48.75±24.97 | .222 | 42.50±26.48 | 45.00±28.32 | .655 |
| SF | 80.95±27.28 | 87.50±22.21 | .222 | 67.50±28.98 | 72.50±29.93 | .458 |
| RQLQ | 1.42±1.13 | 1.03±0.91 | .209 | 1.60±1.10 | 1.72±1.43 | .575 |
BP: body pain; CT: computed tomography; GH: general health; HAD: Hospital Anxiety and Depression scale; IgE: immunoglobulin E; MH: mental health; PF: physical function; ER: emotional role; PR: physical role; RQLQ: Rhinoconjunctivitis Quality of Life Questionnaire; SF: social function; SF-12: abbreviated SF-36 quality of life questionnaire; VT: vitality.
Patients with bad adherence only showed worsening body pain (P=.046).
In the univariate study (Table 4), daytime sleepiness was the only predictive factor for non-compliance (P=.014), and a trend toward poorer compliance was also observed in patients with poorer general health status (P=.059) and better CT 90 (P=.064). In the multivariate analysis, a higher Epworth score was the only independent factor for non-compliance (odds ratio: 1.243; confidence interval: 1.000–1.545]; P<.050).
Clinical, Anatomical, Functional, Biological, Polysomnographic, Therapeutic and Quality of Life Variables Pre-CPAP in Partients with good adherence and Patients with bad adherence.
| Pre-CPAP Variables | Partients with good adherence | Patients with bad adherence | P |
|---|---|---|---|
| Mean±Standard Deviation (n=20) | Mean±Standard Deviation (n=11) | ||
| Age (years) | 57.40±8.83 | 54.55±10.49 | .549 |
| AHI | 44.30±23.12 | 37.27±22.73 | .386 |
| CT 90 | 37.36±25.26 | 17.10±16.90 | .069 |
| Nadir | 67.70±15.94 | 73.29±8.51 | .526 |
| Body mass index | 29.17±3.72 | 29.83±3.02 | .726 |
| Neck circumference (cm) | 43.40±5.21 | 44.36±4.05 | .575 |
| CPAP pressure | 9.15±1.42 | 8.36±0.92 | .148 |
| Hours CPAP | 5.99±1.27 | 2.01±1.35 | .000 |
| Epworth | 11.90±4.70 | 16.73±4.83 | .014 |
| Nasal obstruction | 1.65±1.39 | 1.09±1.13 | .242 |
| Rhinitis symptoms | 1.06±1.11 | 0.55±0.93 | .213 |
| Nasal dryness | 0.00±0.00 | 0.00±0.00 | 1.000 |
| Hearing problems | 0.47±0.70 | 0.36±0.50 | .819 |
| Skin and eye problems | 0.60±1.19 | 0.82±1.25 | .716 |
| HAD | |||
| Anxiety | 7.20±4.14 | 8.00±3.63 | .574 |
| Depression | 4.65±4.12 | 6.09±3.44 | .290 |
| CT (cm3) | 73.81±51.47 | 93.03±58.94 | .397 |
| Otoscopy (normal/abnormal) | 19/1 | 10/1 | 1 |
| Rhinoscopy (normal/abnormal) | 4/16 | 2/9 | 1 |
| Rhinomanometry (cm3/s) | 833.12±301.94 | 834.55±224.46 | .888 |
| Saccharin test (min) | 30.40±23.71 | 32.27±23.12 | .539 |
| Impedanciometry (normal/abnormal) | 19/1 | 11/0 | 1 |
| Audiometry (normal/abnormal) | 20/0 | 11/0 | 1 |
| IgE (U/ml) | 64.56±89.77 | 261.00±648.79 | .962 |
| Eosinophilia (%) | 2.70±1.53 | 2.00±1.00 | .458 |
| Nasal cytology (number of inflammatory cells/500 epithelial cells) | |||
| Eosinophils | 0.50±0.14 | 0.00±0.00 | .184 |
| Neutrophils | 0.76±2.04 | 0.55±0.94 | .629 |
| SF-12 | |||
| GH | 50.71±23.47 | 31.50±26.56 | .059 |
| PF | 73.81±25.59 | 75.00±23.57 | .964 |
| PR | 72.62±22.57 | 65.00±24.15 | .343 |
| ER | 73.61±22.11 | 67.50±24.43 | .444 |
| BP | 79.76±20.34 | 70.00±22.97 | .272 |
| MH | 66.67±20.66 | 57.50±27.13 | .386 |
| VT | 41.67±24.15 | 42.50±26.48 | .947 |
| SF | 80.95±27.28 | 67.50±28.98 | .160 |
| RQLQ | 1.42±1.13 | 1.60±1.10 | .696 |
BP: body pain; CT: computed tomography; CT 90: percentage of time recorded with saturation <90%; GH: general health; HAD: Hospital Anxiety and Depression scale; IgE: immunoglobulin E; MH: mental health; PF: physical function; ER: emotional role; PR: physical role; RQLQ: Rhinoconjunctivitis Quality of Life Questionnaire; SF: social function; SF-12: abbreviated SF-36 quality of life questionnaire; VT: vitality.
The results of this study are surprising and diverge from the proposed hypothesis by showing that while CPAP improves sleepiness and anxiety-depression, it causes, particularly in partients with good adherence, rhinitis, airway dryness, and slower mucociliary clearance. No significant changes were found in other study variables. The only prognostic factor for intolerance was initial sleepiness; none of the ENT variables were prognostic of this outcome.
Some authors consider that the main cause of CPAP intolerance is treatment-related rhinitis, but the published results vary widely. Others have evaluated if patients with CPAP-associated rhinitis improve if the rhinitis is treated, but results were unsatisfactory.24 We were unable to find any evidence that CPAP administered in patients without previous disease causes clinically significant rhinitis, or if this was a reason for therapeutic non-compliance. Nor did we observe any improvement in nasal symptoms in compliant patients.
Previous studies have evaluated the appearance or increase of neutrophils and/or other inflammatory cells in the nasal cytology of patients and CPAP animal models. Again, results varied.25,26 Other authors described specific mediators of nasal inflammation.27 It seems likely that the flow of dry air would stimulate nerve endings to release inflammatory cell mediators28 that could stimulate the secretion of neuropeptides,29 recruiting the neutrophils, thus explaining increased nasal mucosa in CPAP patients.
In a mouse model study performed in our hospital, we showed neutrophilic inflammation after acute application of CPAP.30 Lacedonia et al.,26 however, did not observe any increase in neutrophils in the nasal mucosa of patients receiving CPAP, although nearly all their subjects had elevated neutrophil levels before starting the intervention, suggesting previous nasal pathology. Moreover, in a study recently published by Gelardi et al., the continuous use of CPAP was found to improve rhinitis in SAHS patients, reducing the number of inflammatory cells in nasal cytology.31
In this study, we have confirmed the presence of nasal inflammation, and found it to be more marked in partients with good adherence. Although our results support the presence of nasal cell inflammation, it seems to be generally subclinical if other variables are taken into account. It would be interesting to evaluate if an adaptive phenomenon occurs over time, with improved inflammatory infiltrate, or if, in contrast, inflammatory infiltrate worsens, leading to exacerbation of symptoms.
Assuming that CPAP causes nasal inflammation, this might be reflected in hypertrophy of the mucosa of the nasal cavity causing a reduction of the nasal air space. In our study, analysis of the nasal cavity air volume by CT did not show any significant differences after CPAP. This finding, previously described by Weerdt et al.,32 would suggest that inflammation is mild-moderate. Nasal patency, measured clinically and by rhinomanometry, was not significantly altered in our study, coinciding with Skoczinski et al.,33 who reported that CPAP does not cause nasal obstruction, nor is nasal obstruction a predictive factor for lack of compliance.
Some series have found that nasal dryness affects more than 60% of CPAP patients. We also found this to be true, but it did not interfere with treatment compliance. Humidifiers are usually used to treat dryness, but while they improve symptoms, they do not always improve tolerance or compliance.34,35
In our series, we observed a trend toward worsening mucociliary clearance in partients with good adherence only. This would suggest that changes in mucociliary clearance are due to a time-dependent phenomenon. Similar studies in the literature show contradictory results,36,37 but these did not evaluate the degree of compliance nor mean CPAP pressure.
CPAP obviously provokes an unusual situation of positive pressure within the rhinopharyngeal and oropharygeal cavities.38 This might increase pressure in the middle ear, causing various kinds of symptoms in the ear; some studies even propose CPAP as a possible treatment for ears with mean negative pressures.39,40 However, our results are in line with those of Aksoy et al.,41 who found no significant differences in middle ear pressure. Our auditory assessments, also in line with previous studies,42 revealed no significant auditory changes after treatment.
Predictive Factors for Intolerance to Continuous Positive Airway PressureThe most important finding of our study is the absence of ENT variables as predictive factors for compliance. We only found a higher probability of treatment intolerance in patients with worse general status and greater daytime sleepiness. Thus, the Epworth test result was revealed as the only predictive factor for poor CPAP compliance. This finding is of great interest, since it has also been suggested that patients with greater sleepiness are those who comply best. However, it must be remembered that our patients had no previous nasosinusal pathology, so these results may not be generalizable to other unselected populations. Moreover, while daytime sleepiness was an independent factor for poor compliance, statistical significance was not very high, so our results should be interpreted with caution. Other factors, whether psychological, previous or caused by the SAHS itself, must exist to explain the poorer tolerance in our group.
Predictive factors for CPAP tolerance in the literature vary,43 as there is no accurate method for establishing the degree of treatment compliance. Results are contradictory for AHI and CPAP pressure levels. It has been suggested that high CPAP pressure may be a significant factor, for which reason Sanders and Kem44 and Gulati et al.45 propose that bi-pressure levels may be of benefit. In our study, we found no relationship between pressure and compliance, although we must point out that the mean pressure of our series was not very high (7–12 cmH2O in partients with good adherence and 7–10 cmH2O in patients with bad adherence).
With regard to rhinitis symptoms before CPAP, Farhad et al.46 associated inflammatory markers or neutrophils in nasal mucosa before treatment with CPAP compliance, reporting that patients with more inflammation or clinical symptoms of rhinitis tended to be less compliant. In our study, patients did not have significantly elevated neutrophils in nasal mucosa nor symptoms of rhinitis before starting treatment. Indeed, patients with previous nasal pathology were excluded, so we were only able to determine if patients who presented elevated neutrophils in nasal mucosa after CPAP, even without symptoms, were poorer partients with good adherence – which was not the case. Nor, like Kreivi et al.,47 did we find any relationship between compliance and nasal dryness.
There must be a series of phenomena associated with real or patient-perceived good or bad adaptation that would explain the findings, and other phenomena that complicate CPAP tolerance, ranging from psychological and social considerations to personal beliefs and expectations.48–50
Limitations of our study include, primarily, the small size of our series. Nevertheless, we have used a variety of methods to evaluate different variables for different purposes and studied the parameters at different levels (clinical, anatomical, functional, biological, and polysomnographic), and overall results were consistent. Another limitation of the study is the timepoint (after 2 months of CPAP) at which we made the post-treatment evaluation. Variables such as rhinitis symptoms may subsequently become more severe over time, reflecting increasing neutrophil levels in the nasal mucosa, or else less severe, if neutrophil levels fall due to later adaptation phenomena. The case is the same for daytime sleepiness as a prognostic factor of poor compliance with CPAP treatment; studies with larger numbers of patients or a longer period of CPAP use are needed to consolidate our results.
ConclusionsIn conclusion, CPAP treatment can be generally said to improve a series of clinical parameters, but it causes rhinitis and nasal dryness. The various ENT variables analyzed do not differ greatly between partients with good adherence and patients with bad adherence, and none of them predicts the degree of compliance. Initial sleepiness is the only predictive factor for poor compliance.
The importance of this study is that it is the first to question the generalized impression that adverse ENT effects caused by CPAP impact compliance. There is a need for more larger, randomized, translational studies that also include patients with prior nasal pathology, to clarify the relationship between CPAP and nasal pathology in SAHS patients receiving CPAP.
FundingGrant FISSPI07/0318.
AuthorshipFrancina Aguilar and Ariel Cisternass: study design. Patient visits, ENT examinations, nasal cytology and saccharin test. Coordination of additional tests in all patients, pre- and post-CPAP. Data collection and database construction. Statistical analysis and drafting the manuscript.
Antonio Manuel Ávila and Juan Berenguer: study and calculation of nasal cavity volumes pre- and post-CPAP in all patients and critical review of the manuscript.
Isabel Vilaseca: study design. Clinical follow-up, statistical analysis and drafting the manuscript.
Alejandro Iranzo: patient diagnosis and follow-up and critical review of the manuscript.
Josep Maria Montserrat: patient diagnosis and follow-up and critical review of the manuscript
Marta Torres-López: cytology study and critical review of the manuscript.
Conflict of InterestsThe authors state that they have no conflict of interests.
We would like to thank Maite Carrión, Ainhoa Asensio and Carmen León, for their help in the conduct of this project.
Please cite this article as: Aguilar F, Cisternas A, Montserrat JM, Àvila M, Torres-López M, Iranzo A, et al. Efecto de la presión positiva continua nasal sobre las fosas nasales de pacientes con síndrome de apneas del sueño sin patología nasal previa. Factores predictivos de cumplimiento. Arch Bronconeumol. 2016;52:519–526.