The aim of this study was to investigate the impact of alcohol use disorders (AUD) on community-acquired pneumococcal pneumonia (CAPP) admissions, in terms of in-hospital mortality, prolonged stay and increased hospital spending.
MethodsRetrospective observational study of a sample of CAPP patients from the minimum basic datasets of 87 Spanish hospitals during 2008–2010. Mortality, length of hospital stay and additional spending attributable to AUD were calculated after multivariate covariance analysis for variables such as age and sex, type of hospital, addictions and comorbidities.
ResultsAmong 16,202 non-elective admissions for CAPP in patients aged 18–74years, 2685 had AUD. Patients admitted with CAPP and AUD were predominantly men with a higher prevalence of tobacco or drug use disorders and higher Charlson comorbidity index. Patients with CAPP and AUD had notably higher in-hospital mortality (50.8%; CI 95%: 44.3–54.3%), prolonged length of stay (2.3days; CI 95%: 2.0–2.7days) and increased costs (1869.2€; CI 95%: 1498.6–2239.8€).
ConclusionsAccording to the results of this study, AUD in CAPP patients was associated with increased in-hospital mortality, length of hospital stay and hospital spending.
El objetivo de este estudio es el análisis del impacto de los trastornos asociados al consumo de alcohol (TCA) en las neumonías neumocócicas adquiridas en la comunidad (NNAC), en términos de exceso de mortalidad intrahospitalaria, prolongación de estancias y sobrecostes.
Material y métodosEstudio observacional retrospectivo de una muestra de pacientes que presentaron NNAC recogidos en los conjuntos mínimos básicos de datos de 87 hospitales españoles durante el periodo 2008-2010. Se calculó la mortalidad, la prolongación de estancias y los sobrecostes atribuibles a los TCA controlando mediante análisis multivariado de la covarianza variables como la edad y el sexo, el tipo de hospital, los trastornos adictivos y las comorbilidades.
ResultadosSe estudiaron 16.202 ingresos urgentes por NNAC de 18 a 74años de edad, entre los cuales hubo 2.685 pacientes con TCA. Los ingresos con NNAC y TCA fueron predominantemente varones, con mayor prevalencia de trastornos por tabaco y drogas y con índices de comorbilidad de Charlson más elevados. Los pacientes con NNAC y TCA presentaron importantes excesos de mortalidad (50,8%; IC95%: 44,3-54,3%), prolongación indebida de estancias (2,3días; IC95%: 2,0-2,7días) y sobrecostes (1.869,2€; IC95%: 1.498,6-2.239,8€).
ConclusionesDe acuerdo con los resultados de este estudio, los TCA en pacientes con NNAC aumentan significativamente la mortalidad, la duración de la estancia hospitalaria y sus costes.
Mortality due to infectious diseases in Spain has been falling over the last few decades, but pneumonia remains the primary cause of death in this group (35.9%).1 The most common type of pneumonia, and the one that causes most hospitalizations, is community-acquired pneumococcal pneumonia (CAPP),2,3 the incidence of which remains high in Spain and the rest of Europe.4
Alcohol use disorders (AUDs) are a well-recognized risk factor for CAPP and have an impact on complications and outcomes of patients during admission.5–13 AUDs increase the risk of developing sepsis during pneumonia, a complication that prolongs hospital stay, worsens prognosis14 and increases the rate of unscheduled readmissions.15
We explored this problem in patients aged 18–74 years, admitted to a selected group of 87 Spanish hospitals between 2008 and 2010, and attempted to control for other confounding and interaction factors, such as age, sex, type of hospital, other addictions and comorbidities. The aim of this study was to analyze the potential influence of AUDs on mortality, prolonged stay and increased costs among patients admitted due to CAPP.
MethodsType of Study, Sample and ParticipantsThis was a retrospective, observational study conducted in a selected group of Spanish hospitals.
For the group to be representative of the national situation and the autonomous communities in Spain, 87 hospitals were selected from all the autonomous communities using a stepwise sampling method that took into account the Health Ministry stratification of hospitals according to size and complexity.16
Written or computerized clinical history data were used to code the diagnosis of each patient and the procedures he/she underwent, in accordance with the 9th Revision of the International Classification of Diseases and Causes of Death (ICD-9). Specialist personnel were responsible for coding the data and entering it in the database. These databases, known as minimum basic datasets (MBD), contain demographic information, dates of admission and discharge, type of admission and type of discharge, diagnostic codes for the main and secondary diagnoses, external causes and procedures, classified using ICD-9 codes. They also include diagnosis-related groups (DRGs), and each hospital is categorized according to size and complexity.16
VariablesCases with ICD-9 code 481 in any of the MBD diagnostic codes were defined as cases of CAPP.17,18 Scheduled admissions and patients transferred to another hospital were excluded.
Inclusion in the study was restricted to patients between 18 and 74 years of age. The Charlson comorbidity index19 was calculated as an indicator of comorbidity, using the ICD-9 codes proposed by Quan et al.20 for comorbidities in this index. Other comorbidities were also calculated using the codes proposed by the same authors.20 Alcohol use disorders were defined as any problem associated with the sporadic or chronic excessive consumption of alcohol, identified in the ICD-9 codes as: alcohol dependence (303.00–303.93), alcohol abuse (305.00–305.03), alcohol-induced mental disorders (291.0–291.9), alcoholic polyneuropathy (357.5), alcoholic cardiomyopathy (425.5), alcoholic gastritis (535.30–535.31), alcoholic liver disease (571.0–571.3), excessive blood-alcohol level (790.3), and alcohol poisoning (980.0–980.9 and E860.0-E860.9). ICD-9 codes were also used for the definition of disorders associated with smoking and other addictive drugs.21
Hospitals were divided by size and complexity of care into 5 groups, according to the Ministry of Health, Social Services and Equality classification,16 as required for controlling bias and calculating healthcare costs.
Data AnalysisThe main objective was to determine mortality, duration of stay and hospital costs in patients with CAPP attributable to AUDs. Costs were calculated from specific hospital costs for each DRG, stratified by hospital group, according to the estimates published by the Ministry of Health for the period 2008–2010.16
A bivariate analysis was performed to examine the relationship between CAPP and AUDs, and age, sex, other addictive disorders and comorbidities, using the Chi-squared test (or non-parametric variants of this test) and the Student's t-test (or non-parametric variants). To minimize the confounding bias, a multivariate analysis of covariance was performed to determine the effect of AUDs on CAPP patients with regard to in-hospital mortality, stay and costs. The requirements of the continuous variables were verified after identifying the best-fit model, and data were adjusted for age, sex, addictions, hospital group, and patient severity (according to the Charlson index). Statistical significance was set at <.0001, due to the sample size and the multiple comparison performed. The adjusted mean of each of the dependent variables (mortality, days of hospital stay, and costs on discharge) was calculated for CAPP patients with and without AUDs, and differences were measured. The analysis was performed using the STATA statistical package, version MP 13.1.
The recommendations of the STROBE guidelines for observational studies were followed in the design, analysis and presentation of results, as applicable.
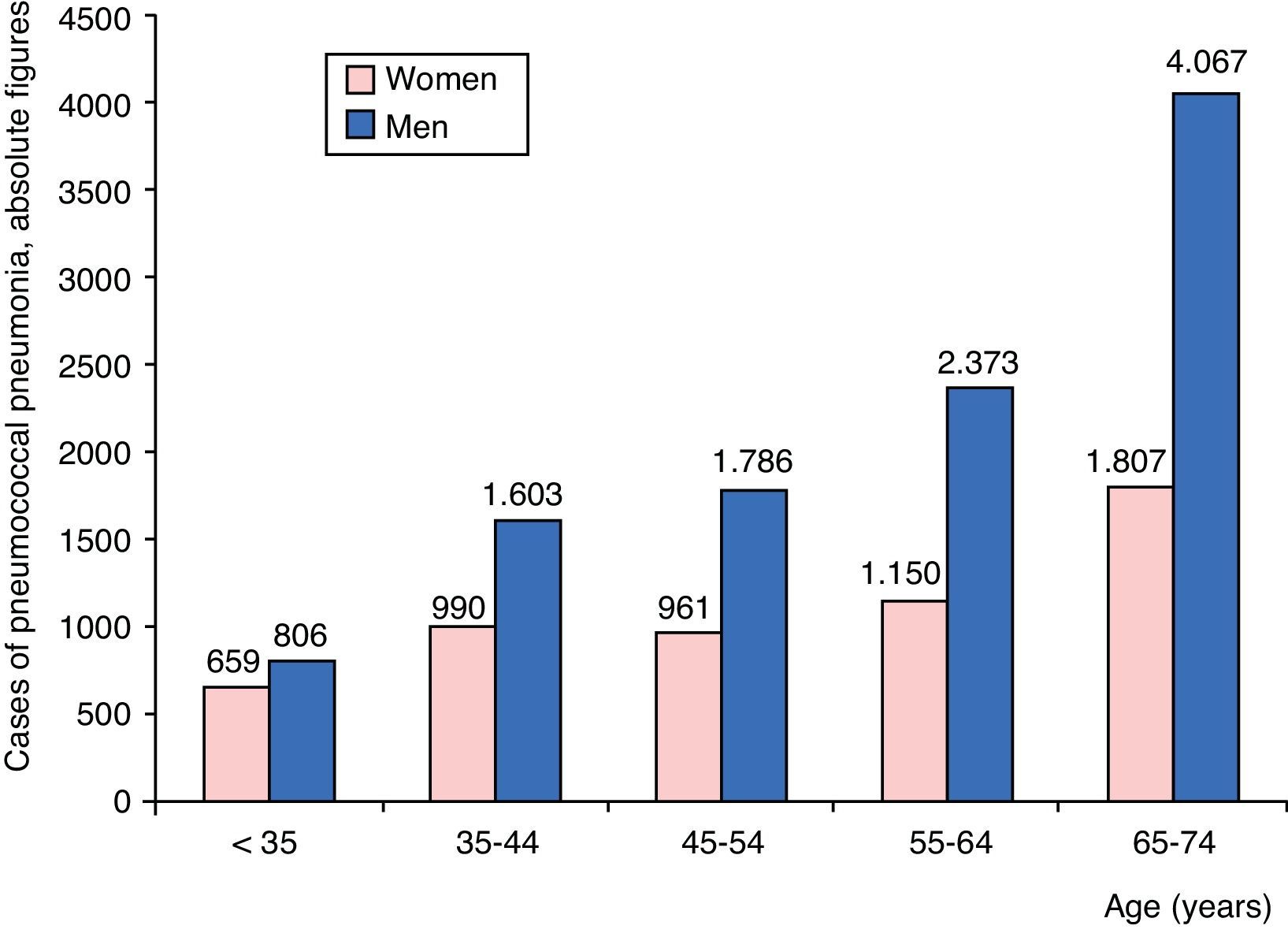
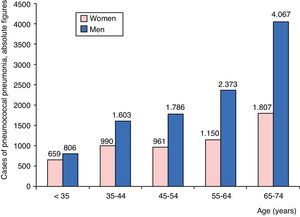
ResultsPatient CharacteristicsAt total of 16,202 admissions for CAPP were identified: 10,635 men (65.6%) and 5567 women (34.4%). Distribution by age group and sex of patients admitted for CAPP are shown in Fig. 1. The number of admissions can be seen to rise progressively with age, primarily in men, until the age of 74 years.
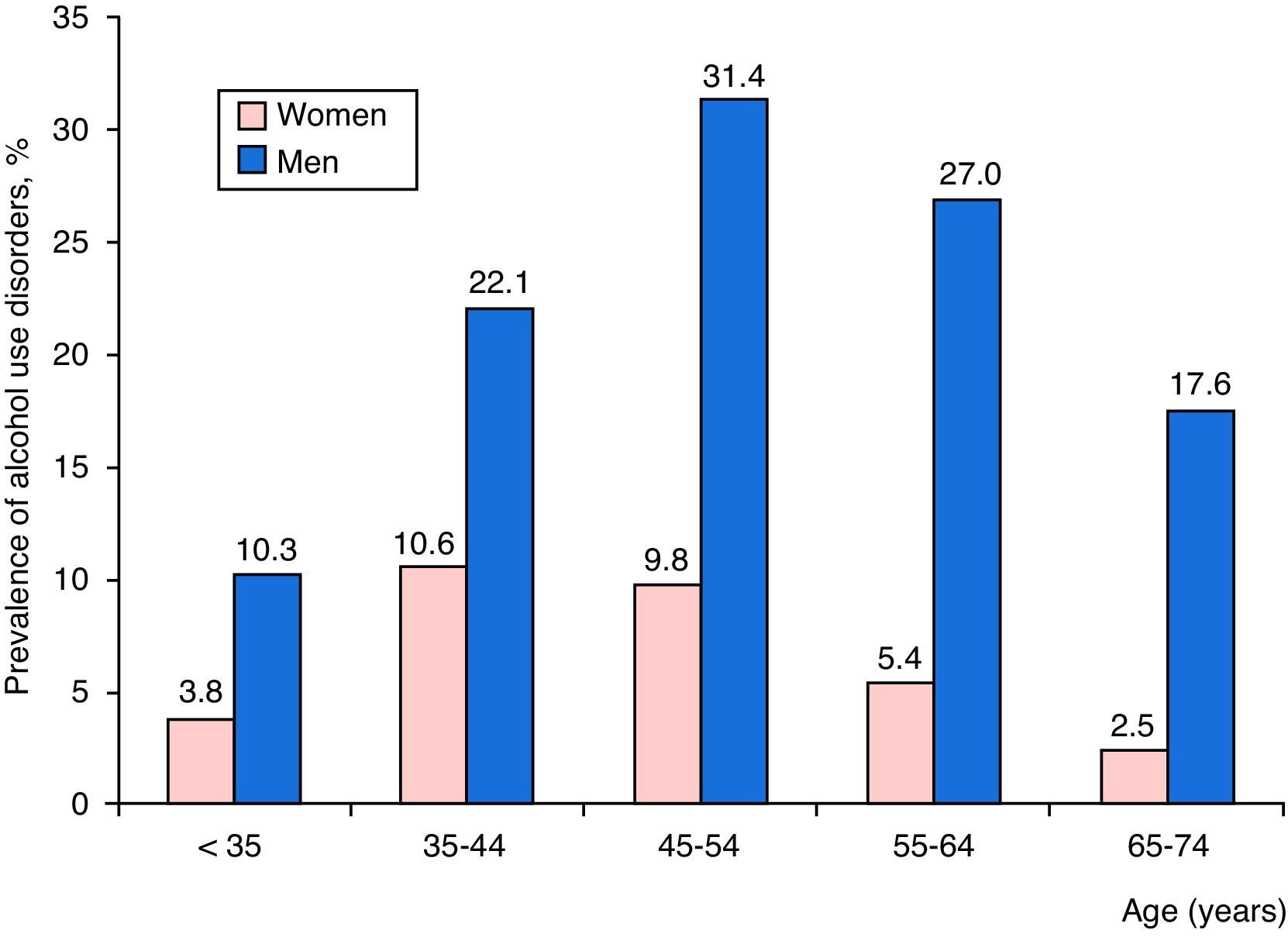
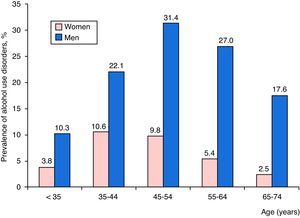
A total of 2685 patients with CAPP had AUDs (16.6%), and differences between the sexes were notable: 2353 men (22.1%) and 332 women (6.0%). Distribution by age and sex of admissions for CAPP with AUDs is shown in Fig. 2, where it can be seen that AUDs were more common among men, mainly in the 45–54 years age group, followed by those aged 55–64, and then 35–44. The highest prevalence of AUDs among women was found in the 35–44 year age group, followed by those aged 45–54, and 55–64.
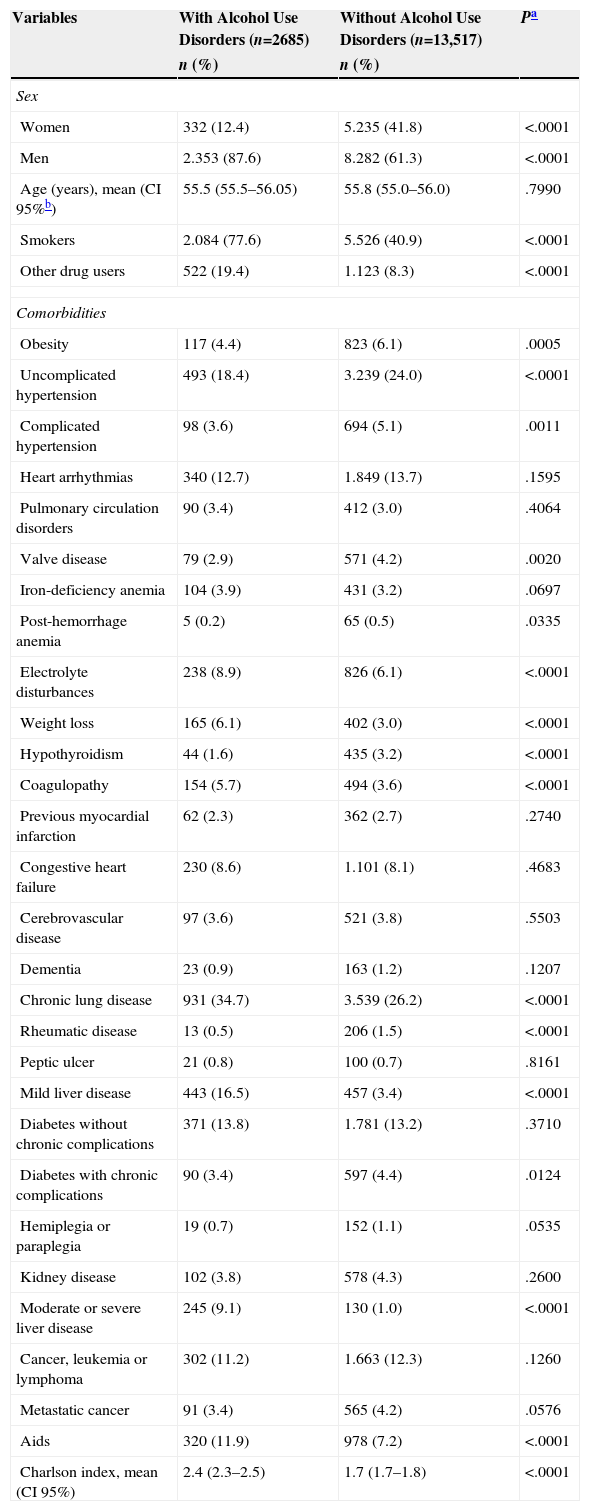
The characteristics of CAPP patients with and without AUDs are shown in Table 1. Patients with AUDs were mainly men, and had a higher rate of other addictions, mainly smoking (77.6%) and other drugs (19.4%). They also presented a higher prevalence of some of the comorbidities studied on admission, such as electrolyte disturbances, weight loss, coagulopathies, chronic lung disease, liver disease and AIDS, and a higher Charlson comorbidity index score.
Characteristics of patients with community-acquired pneumococcal pneumonia with and without alcohol use disorders.
| Variables | With Alcohol Use Disorders (n=2685) | Without Alcohol Use Disorders (n=13,517) | Pa |
|---|---|---|---|
| n (%) | n (%) | ||
| Sex | |||
| Women | 332 (12.4) | 5.235 (41.8) | <.0001 |
| Men | 2.353 (87.6) | 8.282 (61.3) | <.0001 |
| Age (years), mean (CI 95%b) | 55.5 (55.5–56.05) | 55.8 (55.0–56.0) | .7990 |
| Smokers | 2.084 (77.6) | 5.526 (40.9) | <.0001 |
| Other drug users | 522 (19.4) | 1.123 (8.3) | <.0001 |
| Comorbidities | |||
| Obesity | 117 (4.4) | 823 (6.1) | .0005 |
| Uncomplicated hypertension | 493 (18.4) | 3.239 (24.0) | <.0001 |
| Complicated hypertension | 98 (3.6) | 694 (5.1) | .0011 |
| Heart arrhythmias | 340 (12.7) | 1.849 (13.7) | .1595 |
| Pulmonary circulation disorders | 90 (3.4) | 412 (3.0) | .4064 |
| Valve disease | 79 (2.9) | 571 (4.2) | .0020 |
| Iron-deficiency anemia | 104 (3.9) | 431 (3.2) | .0697 |
| Post-hemorrhage anemia | 5 (0.2) | 65 (0.5) | .0335 |
| Electrolyte disturbances | 238 (8.9) | 826 (6.1) | <.0001 |
| Weight loss | 165 (6.1) | 402 (3.0) | <.0001 |
| Hypothyroidism | 44 (1.6) | 435 (3.2) | <.0001 |
| Coagulopathy | 154 (5.7) | 494 (3.6) | <.0001 |
| Previous myocardial infarction | 62 (2.3) | 362 (2.7) | .2740 |
| Congestive heart failure | 230 (8.6) | 1.101 (8.1) | .4683 |
| Cerebrovascular disease | 97 (3.6) | 521 (3.8) | .5503 |
| Dementia | 23 (0.9) | 163 (1.2) | .1207 |
| Chronic lung disease | 931 (34.7) | 3.539 (26.2) | <.0001 |
| Rheumatic disease | 13 (0.5) | 206 (1.5) | <.0001 |
| Peptic ulcer | 21 (0.8) | 100 (0.7) | .8161 |
| Mild liver disease | 443 (16.5) | 457 (3.4) | <.0001 |
| Diabetes without chronic complications | 371 (13.8) | 1.781 (13.2) | .3710 |
| Diabetes with chronic complications | 90 (3.4) | 597 (4.4) | .0124 |
| Hemiplegia or paraplegia | 19 (0.7) | 152 (1.1) | .0535 |
| Kidney disease | 102 (3.8) | 578 (4.3) | .2600 |
| Moderate or severe liver disease | 245 (9.1) | 130 (1.0) | <.0001 |
| Cancer, leukemia or lymphoma | 302 (11.2) | 1.663 (12.3) | .1260 |
| Metastatic cancer | 91 (3.4) | 565 (4.2) | .0576 |
| Aids | 320 (11.9) | 978 (7.2) | <.0001 |
| Charlson index, mean (CI 95%) | 2.4 (2.3–2.5) | 1.7 (1.7–1.8) | <.0001 |
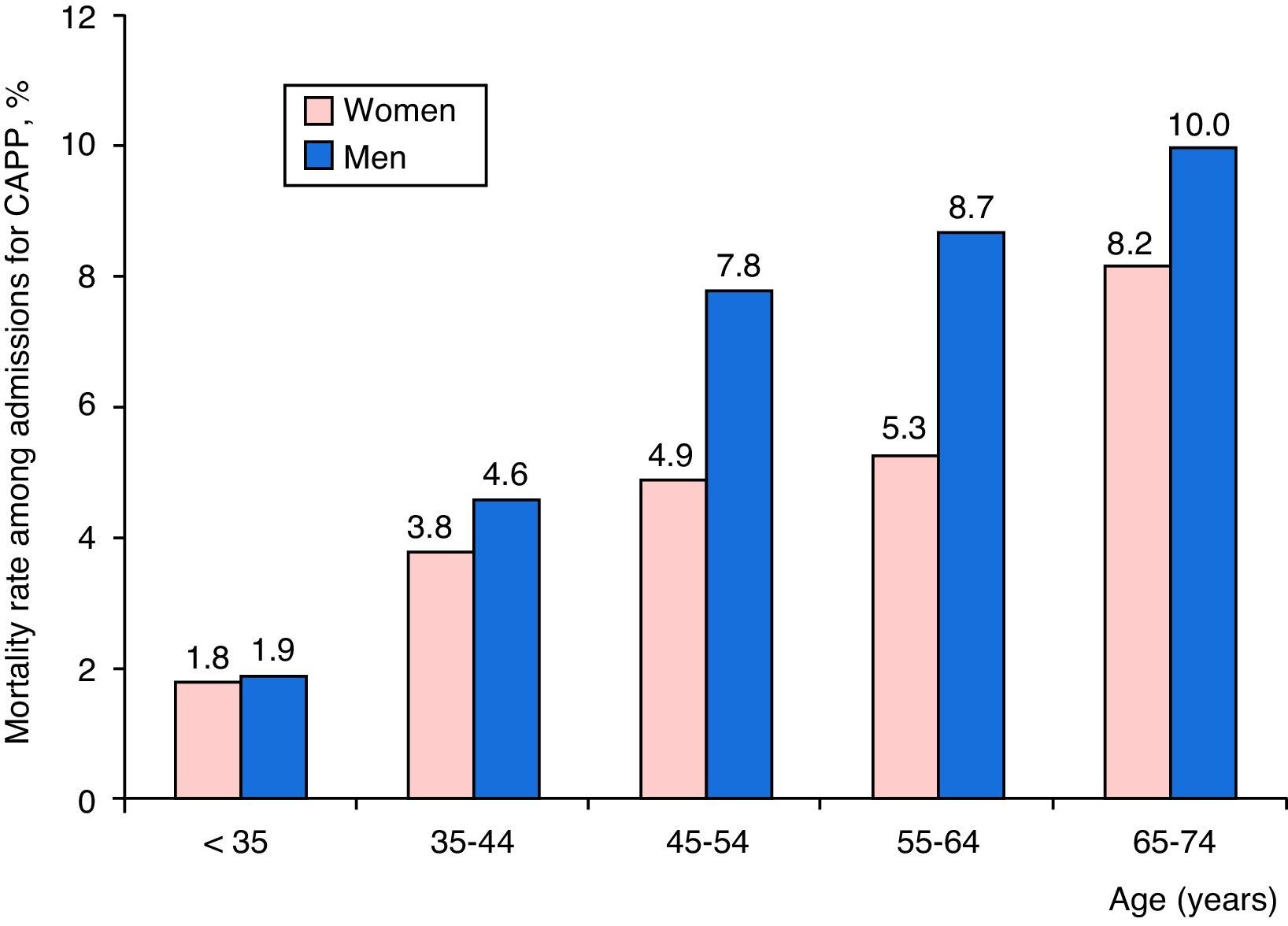
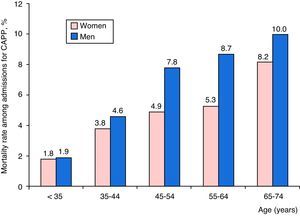
Distribution of mortality among CAPP patients according to age group and sex is shown in Fig. 3. Death rates were higher among men, and increased progressively with age.
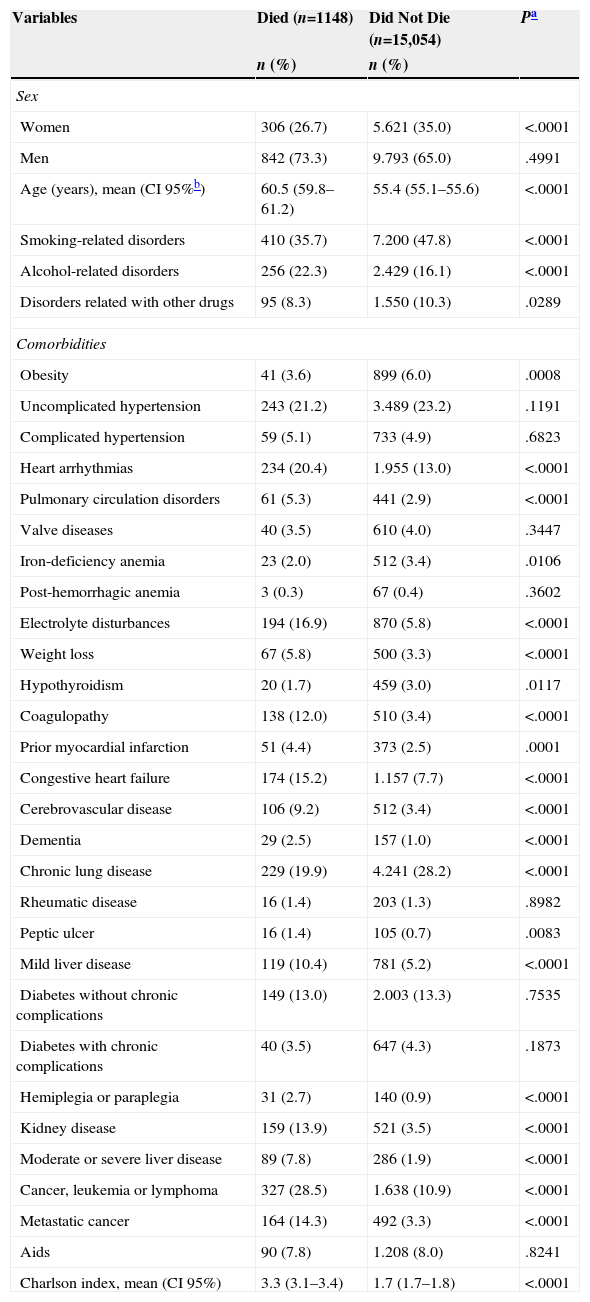
The characteristics of patients admitted for CAPP who died and did not die during admission are shown in Table 2. CAPP patients who died were older (mean age, 60.5 years) and mainly men (73.3% of those who died). Some comorbidities were also found more frequently on admission, such as arrhythmias, pulmonary circulation disorders, electrolyte disturbances, weight loss, coagulopathies, congestive heart failure, cerebrovascular disease, dementia, liver disease, hemiplegia or paraplegia, kidney disease, cancer, leukemia, lymphoma and metastatic cancer, and a higher Charlson comorbidity index score.
Characteristics of Patients Admitted for Community-Acquired Pneumococcal Pneumonia Who Died or Did Not Die During Hospitalization.
| Variables | Died (n=1148) | Did Not Die (n=15,054) | Pa |
|---|---|---|---|
| n (%) | n (%) | ||
| Sex | |||
| Women | 306 (26.7) | 5.621 (35.0) | <.0001 |
| Men | 842 (73.3) | 9.793 (65.0) | .4991 |
| Age (years), mean (CI 95%b) | 60.5 (59.8–61.2) | 55.4 (55.1–55.6) | <.0001 |
| Smoking-related disorders | 410 (35.7) | 7.200 (47.8) | <.0001 |
| Alcohol-related disorders | 256 (22.3) | 2.429 (16.1) | <.0001 |
| Disorders related with other drugs | 95 (8.3) | 1.550 (10.3) | .0289 |
| Comorbidities | |||
| Obesity | 41 (3.6) | 899 (6.0) | .0008 |
| Uncomplicated hypertension | 243 (21.2) | 3.489 (23.2) | .1191 |
| Complicated hypertension | 59 (5.1) | 733 (4.9) | .6823 |
| Heart arrhythmias | 234 (20.4) | 1.955 (13.0) | <.0001 |
| Pulmonary circulation disorders | 61 (5.3) | 441 (2.9) | <.0001 |
| Valve diseases | 40 (3.5) | 610 (4.0) | .3447 |
| Iron-deficiency anemia | 23 (2.0) | 512 (3.4) | .0106 |
| Post-hemorrhagic anemia | 3 (0.3) | 67 (0.4) | .3602 |
| Electrolyte disturbances | 194 (16.9) | 870 (5.8) | <.0001 |
| Weight loss | 67 (5.8) | 500 (3.3) | <.0001 |
| Hypothyroidism | 20 (1.7) | 459 (3.0) | .0117 |
| Coagulopathy | 138 (12.0) | 510 (3.4) | <.0001 |
| Prior myocardial infarction | 51 (4.4) | 373 (2.5) | .0001 |
| Congestive heart failure | 174 (15.2) | 1.157 (7.7) | <.0001 |
| Cerebrovascular disease | 106 (9.2) | 512 (3.4) | <.0001 |
| Dementia | 29 (2.5) | 157 (1.0) | <.0001 |
| Chronic lung disease | 229 (19.9) | 4.241 (28.2) | <.0001 |
| Rheumatic disease | 16 (1.4) | 203 (1.3) | .8982 |
| Peptic ulcer | 16 (1.4) | 105 (0.7) | .0083 |
| Mild liver disease | 119 (10.4) | 781 (5.2) | <.0001 |
| Diabetes without chronic complications | 149 (13.0) | 2.003 (13.3) | .7535 |
| Diabetes with chronic complications | 40 (3.5) | 647 (4.3) | .1873 |
| Hemiplegia or paraplegia | 31 (2.7) | 140 (0.9) | <.0001 |
| Kidney disease | 159 (13.9) | 521 (3.5) | <.0001 |
| Moderate or severe liver disease | 89 (7.8) | 286 (1.9) | <.0001 |
| Cancer, leukemia or lymphoma | 327 (28.5) | 1.638 (10.9) | <.0001 |
| Metastatic cancer | 164 (14.3) | 492 (3.3) | <.0001 |
| Aids | 90 (7.8) | 1.208 (8.0) | .8241 |
| Charlson index, mean (CI 95%) | 3.3 (3.1–3.4) | 1.7 (1.7–1.8) | <.0001 |
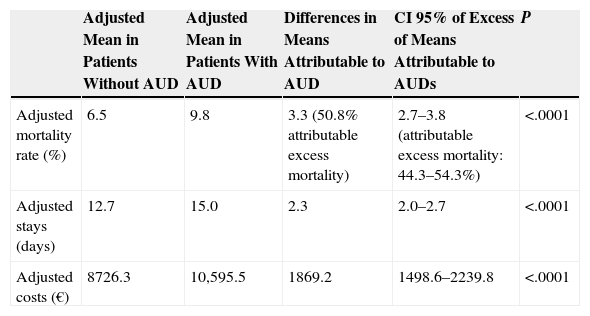
Results of the multivariate analysis of covariance, which included age, sex, hospital group, all addictions, and the Charlson comorbidity index, are shown in Table 3. Of the 2685 CAPP patients with AUDs, 256 died (crude mortality rate; 9.5%), compared to 892 of the 13,517 CAPP patients without AUDs (6.6%). In the multivariate model, adjusted mean mortality rates were significantly higher among CAPP admissions with AUDs (9.8% vs 6.5%), with a 3.3% mean difference, representing an excess of AUD-associated mortality of 50.8%.
Death During Hospitalization, Prolongation of Stay and Additional Costs Attributable to Alcohol Use Disorders in Patients With Community-Acquired Pneumococcal Pneumonia.a
| Adjusted Mean in Patients Without AUD | Adjusted Mean in Patients With AUD | Differences in Means Attributable to AUD | CI 95% of Excess of Means Attributable to AUDs | P | |
|---|---|---|---|---|---|
| Adjusted mortality rate (%) | 6.5 | 9.8 | 3.3 (50.8% attributable excess mortality) | 2.7–3.8 (attributable excess mortality: 44.3–54.3%) | <.0001 |
| Adjusted stays (days) | 12.7 | 15.0 | 2.3 | 2.0–2.7 | <.0001 |
| Adjusted costs (€) | 8726.3 | 10,595.5 | 1869.2 | 1498.6–2239.8 | <.0001 |
The same table shows that means adjusted for hospital stay were significantly higher among CAPP admissions with AUDs (15.0 vs 12.7 days), with a mean AUD-associated extended stay of 2.3 days.
Finally, means adjusted for costs of hospital stay were significantly higher among CAPP admissions with AUDs (€10,595.50 vs €8726.30), representing an additional cost of €1869.20 for each AUD-associated CAPP.
DiscussionPneumococcus remains the most common causative agent of community-acquired pneumonia. In the United States, however, the rate has declined, and is now only detected in 10%–15% of hospitalized cases. This reduction has been attributed to the combined influence of various factors,22 such as widespread administration of the pneumococcal polysaccharide vaccine in adults,23 almost universal administration of the pneumococcal conjugated vaccine in children,24 and falling smoking rates.25 In Spain and other European countries where these vaccines have been used less and where high smoking rates persist, pneumococcus is still responsible for the greatest proportions of community-acquired pneumonia.4,26
The results obtained in this study indicate that AUDs have a considerable impact on in-hospital mortality in CAPP patients, causing significantly prolonged hospital stays and generating additional costs per discharged patient. Both occasional excessive consumption of alcohol and chronic alcohol abuse or dependency syndromes cause serious disturbances in specific and non-specific immunity, generating a risk factor not only for CAPP but also for the serious complications observed in these patients.11,14,27
Due to size of the sample and the diversity of the hospitals in this study, our results are generalizable and need not be limited to patients from one or a few hospitals. This, to the best of our knowledge, is the first study conducted in Spain that calculates excess mortality, prolonged stay and additional costs attributable to AUDs in CAPP patients.
The major challenge in analyzing the influence of AUDs on the prognosis and other outcomes of hospitalized patients is adequate control of the confounding bias. Length of stay, costs, and in-hospital mortality differ depending on the reason for admission, the severity of the disease, comorbidities, type of hospital, and other demographic and social patient characteristics.28 Including the hospital group in the multivariate model for controlling the confounding bias is very important, since scientific evidence has shown that quality of medical care and outcomes differ depending on hospital type, facilities and standards of care.1,6,7,10
Our study has several limitations. Our data were sourced exclusively from the MBDs, and no additional patient data were provided. Throughout the study, the definitions of addictive disorders, CAPP and comorbidities were used exactly as assigned by the doctors in each hospital, and coded and entered into the databases by the data managers, with no knowledge of interhospital variability. ICD-9 codes for identifying CAPP are those internationally used for studies based on hospital discharge databases, but there is no means of corroborating these diagnoses with any clinical, radiological and laboratory criteria for diagnosing CAPP that may be mentioned in the clinical records. Previous studies have found that ICD-9 code 481 is highly sensitive and highly specific for hospitalized cases of CAPP if all and not only the principal diagnostic codes are included. This is because CAPP patients are frequently admitted with sepsis, respiratory failure or other diagnoses that are coded as the principal diagnosis, and the diagnosis of CAPP is relegated to one of the secondary diagnoses.17,18,29 To avoid this data bias, all diagnostic codes were taken into consideration in this study, not only the principal diagnosis. Moreover, patients with programmed admission were excluded to reduce as far as possible the inclusion of cases with possibly nosocomial pneumococcal pneumonia.
Databases such as MBDs also have notable advantages. The data collected is usually entered in nearly all hospital discharge records. As all cases are included, quite accurate estimates can be made on incidence, prevalence, comorbidities, complications, and mortality of the diseases seen.9,30,31 These data can be analyzed retrospectively, unlike other designs that need prospective data collection. Data can be collected relatively quickly and easily over long periods and from a large number of patients, as was the case in our study, and such systematic collection considerably reduces costs. In these studies, less selection bias may be generated than in studies in which patients or their legal representatives may refuse to provide written informed consent to participate. Another considerable advantage lies in the availability of defined costs for each DRG stratified by hospital group and year, which facilitates the calculation of excess costs due to CAPP and AUDs.
A consensus document produced by several Spanish scientific societies recommends anti-pneumococcal vaccination in adults with underlying disease, including patients with AUDs.32 This recommendation should be following in all care settings, including hospital departments in which patients with AUDs are identified.
The results of this study are a reminder that the diagnosis of alcohol, tobacco and drug abuse and the introduction of therapy should be one of the main therapeutic objectives prior to discharge of CAPP patients. Investigating the alcohol and drug use and smoking habit of each patient is the ethical and professional duty of each physician. Strategies such as a brief talk on the risks of alcohol, smoking and drugs, and a note in the discharge report advising the primary care physician of the problem in case the patient needs to be referred to specialized detoxification units, have been shown to be effective33–36 in preventing complications and readmissions. Reducing the number of admissions and readmissions attributable to these disorders would help to reduce the costs of sick leave from work and hospital stays, increase the availability of beds in hospitals and reduce the risk of death. Each case of CAPP associated with alcohol, smoking or drug addiction disorders that is avoided also reduces the overall burden borne by these patients and their families.
ConclusionsIn patients admitted for CAPP, AUDs account for an excess in-hospital mortality of 50.8%, prolongation of stay of 2.3 days, and excess costs of €1869.20.
FundingThis study was funded by: (a) the Government Delegation for the National Drug Plan. Ministry of Health, Social Services and Equality (Grant No. 2009I017, Project G41825811), and (b) Subsidies for Funding Biomedical and Health Science Research in Andalusia for 2013. Department of Health and Social Affairs of the Government of Andalusia (PI-0271-2013).
Authors’ ContributionAll authors are equally contributed to the study design, data analysis and interpretation, drafting and review of the article and approval of final version.
Conflict of InterestsThe authors state that they had no conflict of interests.
Please cite this article as: Gili-Miner M, López-Méndez J, Béjar-Prado L, Ramírez-Ramírez G, Vilches-Arenas Á, Sala-Turrens J. Trastornos por consumo de alcohol y neumonía neumocócica adquirida en la comunidad: mortalidad atribuible, prolongación de estancias y sobrecostes hospitalarios. Arch Bronconeumol. 2015;51:564–570.