The magnitude of current resistance to antituberculosis drugs in Spain is unknown. The objective of this study is to describe resistance to first-line antituberculosis drugs and determine the associated factors.
MethodsProspective multicenter study of adult tuberculosis patients with positive Mycobacterium tuberculosis culture and antibiogram including first-line drugs in 32 hospitals and one out-patient center of the Spanish Health System between 2010 and 2011.
ResultsA total of 519 patients, 342 Spanish nationals and 177 (34.1%) foreigners were studied. Drug resistance was found in 48 (9.2%), of which 35 (6.7%) were isoniazid-resistant. There were 10 (1.9%) multiresistant cases and no strain was extremely resistant. Initial isoniazid resistance was detected in 28 of the 487 (5.7%) antituberulosis-naïve patients, most of whom were foreigners (p<.01). Acquired resistance was seen in 7 (22.6%) previously treated cases. Multiresistance was initial in 6 cases (1.2%) and acquired in another 4 (12.9%). Factors associated with initial isoniazid resistance were immigrant status and group cohabitation (OR=2.3; 95% CI: 0.98–5.67 and OR=2.2; 95% CI: 1.05–7.07 respectively). The factor associated with acquired resistance to isoniazid was age below 50 years (p=.03).
ConclusionsThe rate of initial isoniazid resistance is greater than estimated, probably due to the increase in immigration during recent years, suggesting that systematic national monitoring is required. Immigrants and those who cohabit in groups have a higher risk of isoniazid resistance.
La magnitud de la resistencia actual a fármacos antituberculosos en España es desconocida. El objetivo del estudio es describir la resistencia a fármacos antituberculosos de primera línea y determinar sus factores asociados.
MétodosEstudio prospectivo multicéntrico de pacientes tuberculosos adultos con aislamiento de Mycobacterium tuberculosis y antibiograma de fármacos de primera línea en 32 hospitales y un centro extrahospitalario del sistema sanitario nacional durante los años 2010 y 2011.
ResultadosSe estudió a 519 pacientes, 342 españoles y 177 (34,1%) extranjeros, 48 de ellos (9,2%) con resistencia a cualquier fármaco, de los que 35 (6,7%) eran resistentes a isoniacida. Hubo 10 casos multirresistentes (1,9%) y ninguno extremadamente resistente. Se detectó resistencia inicial a isoniacida en 28 de los 487 (5,7%) pacientes sin antecedentes de tratamiento antituberculoso previo, afectando más a los extranjeros (p<0,01), y resistencia adquirida en 7 (22,6%) casos previamente tratados. La multirresistencia fue inicial en 6 casos (1,2%) y adquirida en otros 4 (12,9%). Los factores asociados a tener resistencia inicial a isoniacida fueron ser inmigrante y la convivencia en grupo (OR=2,3; IC del 95%, 0,98–5,67, y OR=2,2; IC del 95%, 1,05–7,07, respectivamente). El factor asociado a la existencia de resistencia adquirida a isoniacida fue la edad inferior a 50 años (p=0,03).
ConclusionesLa tasa de resistencia inicial a isoniacida es superior a la estimada, probablemente debida al aumento de la inmigración durante los últimos años, lo que aconseja su vigilancia nacional sistemática. Los individuos inmigrantes y los que conviven en grupo tienen mayor riesgo de tener resistencia a isoniacida.
The World Health Organization (WHO) estimates that in 2011 there were 8.7 million (125/100000 population) new cases of tuberculosis (TB), 13% of which were co-infected with HIV. Global deaths from TB have fallen by 41% since 1990,1 while a decrease is also expected in morbidity due to a treatment compliance rate of over 85% in new cases and 87% in smear-positive patients.1 This hope could be dashed, however, because of an increase in cases of drug-resistant TB (DR-TB), multidrug-resistant TB (MDR-TB) and extensively drug-resistant TB (XDR-TB) caused by Mycobacterium tuberculosis (MT). It is estimated that 3.7% of new cases and 20% of previously treated cases have MDR-TB, with XDR-TB accounting for 9% of MDR-TB cases reported.1 In 1994, the WHO and the International Union Against Tuberculosis and Lung Disease (IUATLD) launched their Global Project on Anti-tuberculosis Drug Resistance Surveillance, collecting standardized worldwide data on MT strains studied in expressly accredited microbiology laboratories.2 Their latest report, based on data from 2002 to 2007, estimated a mean prevalence of DR-TB of 11.1% and a prevalence of MDR-TB of 1.6% in never-treated patients. A subsequent report concluded that the highest global rates of MDR-TB in history were recorded in 2009 and 2010 (65.1% in Moldavia).5
The resistance rates detected in 2005 in the only 3 Spanish regions to participate in the program (Barcelona, Galicia, and Aragón) varied: 7.3%–9.9% for DR-TB; 3.9%–7.1% for isoniazid-resistant TB (DR-TB-H) and 0.3%–1.8% for MDR-TB.3–5 The European Centre for Disease Prevention and Control, based on Spanish data from 2010 with drug susceptibility testing (DST) carried out in 35.5% of cases, published DR-TB-H rates of 7.7% and MDR-TB rates of 3.5%, of which 6.1% were XDR-TB.6 Two representative studies in the Spanish TB population describe similar results with the following rates: DR-TB 7.9%–8.3%, DR-TB-H close to 5%, and few cases of MDR-TB in never-treated patients.7,8
The aim of our study was to prospectively estimate resistance to first-line antituberculosis (anti-TB) drugs in Spain in a cohort of TB patients, by means of systematic MT culture and DST.
MethodsThe RETUBES study is a prospective, multicenter observational study of a cohort of patients diagnosed with TB in Spain between January 1, 2010 and December 31, 2011, in whom MT was isolated and DST carried out. The study was designed by the Comprehensive Program for Research in Tuberculosis (PII-TB) of the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR), and offered to all its members with the support of the PII-TB infrastructure and a SEPAR 2008 research grant.
A total of 519 adult patients who agreed to participate in the study were included. They were diagnosed with TB in 32 hospitals and 1 outpatient unit in 13 Spanish regions by pulmonologists and infectious disease specialists from the PII-TB. The patient inclusion criteria were: age 18 years or over, MT isolates, DST for first-line drugs, and patient informed consent to use their data. In order to characterize the patients according to previous history of anti-TB treatment, 1 case was excluded due to loss of data.
MT was isolated in each center from cultures grown on solid Lowenstein–Jensen (L–J) medium or in liquid medium subsequently confirmed by L–J, as per their standard laboratory method. DST was performed in tertiary hospitals, again as per their standard laboratory method, while samples from the remaining hospitals were referred to national reference centers. Drug sensitivity was studied using conventional phenotypic methods, either on solid medium using the indirect proportions method, or in liquid medium using the automated BACTEC™ MGIT 960 system (Becton Dickinson Diagnostic Systems, Sparks, MD, USA).
DefinitionsDR-TB: TB with MT resistance to any first-line anti-TB drug.
DR-TB-H: TB with MT resistance to H.
MDR-TB: TB with MT resistance to at least both H and rifampicin (R).
XDR-TB: MDR-TB with additional MT resistance to any fluoroquinolone and at least 1 of 3 second-line anti-TB drugs (capreomycin, kanamycin or amikacin).
Primary resistance: MT resistance to 1 or more drugs before beginning treatment of a new patient who has never been treated with anti-TB drugs.
Acquired resistance: MT resistance to 1 or more drugs in patients treated for 1 or more months with anti-TB drugs.
Foreign-born: in this study, the TB cases correspond to economic immigrants from developing countries.
VariablesData were collected and recorded in an electronic case report form filled out on the website for subsequent data cleaning and analysis. The primary variable was resistance to first-line anti-TB drugs, while the secondary variables included the epidemiological, microbiological and clinical characteristics.
Statistical AnalysisA descriptive study of the prevalence of drug-resistant MT was carried out by frequency distribution of qualitative variables. Bivariate analysis was performed using the χ2 test, with Fisher's two-tailed test and Yates correction when the expected values were less than 5. The risk factors for DR-TB-H in never-treated patients were analyzed by logistic regression (stepwise method), including exploratory analysis of all risk factors that could have an effect at bivariate level. The Hosmer and Lemeshow test was used to check the goodness-of-fit of the model. A P value <.05 was considered statistically significant. Factors associated with DR-TB-H in previously treated patients could only be analyzed at bivariate level, as they had insufficient statistical power at multivariate level. As an association measure, the odds ratio (OR) was calculated, with 95% confidence intervals (95% CI). All analyses were performed using the statistical package SPSS v. 18.0 (SPSS Inc, Chicago, USA).
The project was approved by the Hospital Universitario Dr. Peset (Valencia) Clinical Research Ethics Committee (CREC) and the CRECs of another 12 national hospitals. All records containing patient identification data were treated confidentially in accordance with Spanish Law 15/1999 on Personal Data Protection.
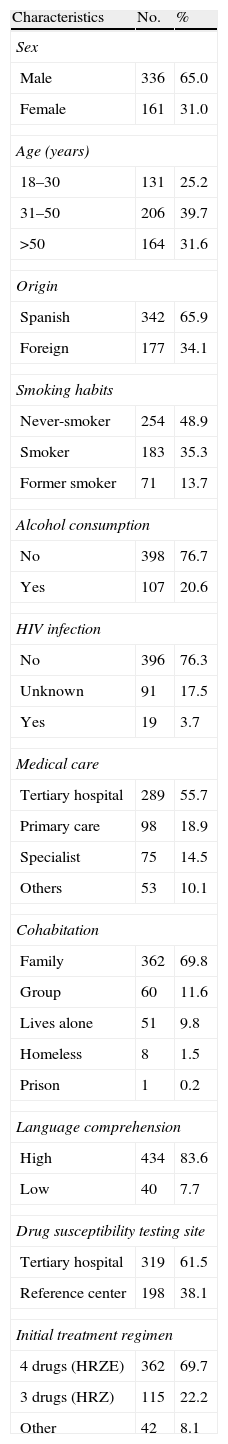
ResultsA total of 554 tuberculosis patients with positive MT culture were initially included, but the final study sample was restricted to the 519 patients with DST results, 342 (65.9%) Spanish-born and 177 (34.1%) foreign-born. The sociodemographic and clinical characteristics are described in Table 1.
Sociodemographic and Clinical Characteristics of the 519 Tuberculosis Patients Included in the RETUBES Study.
| Characteristics | No. | % |
| Sex | ||
| Male | 336 | 65.0 |
| Female | 161 | 31.0 |
| Age (years) | ||
| 18–30 | 131 | 25.2 |
| 31–50 | 206 | 39.7 |
| >50 | 164 | 31.6 |
| Origin | ||
| Spanish | 342 | 65.9 |
| Foreign | 177 | 34.1 |
| Smoking habits | ||
| Never-smoker | 254 | 48.9 |
| Smoker | 183 | 35.3 |
| Former smoker | 71 | 13.7 |
| Alcohol consumption | ||
| No | 398 | 76.7 |
| Yes | 107 | 20.6 |
| HIV infection | ||
| No | 396 | 76.3 |
| Unknown | 91 | 17.5 |
| Yes | 19 | 3.7 |
| Medical care | ||
| Tertiary hospital | 289 | 55.7 |
| Primary care | 98 | 18.9 |
| Specialist | 75 | 14.5 |
| Others | 53 | 10.1 |
| Cohabitation | ||
| Family | 362 | 69.8 |
| Group | 60 | 11.6 |
| Lives alone | 51 | 9.8 |
| Homeless | 8 | 1.5 |
| Prison | 1 | 0.2 |
| Language comprehension | ||
| High | 434 | 83.6 |
| Low | 40 | 7.7 |
| Drug susceptibility testing site | ||
| Tertiary hospital | 319 | 61.5 |
| Reference center | 198 | 38.1 |
| Initial treatment regimen | ||
| 4 drugs (HRZE) | 362 | 69.7 |
| 3 drugs (HRZ) | 115 | 22.2 |
| Other | 42 | 8.1 |
HIV: human immunodeficiency virus.
The difference between 519 and the total figures for each variable corresponds to cases in which the datum was not recorded.
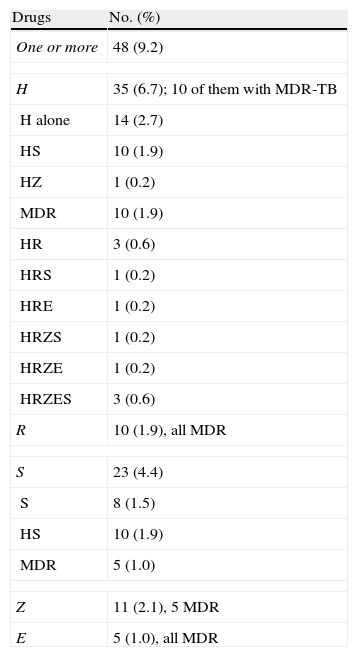
DR-TB was detected in 48 (9.2%) patients: 35 (6.7%) were H-resistant, 10 (1.9%) were R-resistant (all MDR-TB) and the remaining patients were resistant to other drugs (Table 2). H-resistance was most common in foreign-born patients (Table 3).
Antituberculosis Drug Resistance in the 519 Cases Included in the RETUBES Study.
| Drugs | No. (%) |
| One or more | 48 (9.2) |
| H | 35 (6.7); 10 of them with MDR-TB |
| H alone | 14 (2.7) |
| HS | 10 (1.9) |
| HZ | 1 (0.2) |
| MDR | 10 (1.9) |
| HR | 3 (0.6) |
| HRS | 1 (0.2) |
| HRE | 1 (0.2) |
| HRZS | 1 (0.2) |
| HRZE | 1 (0.2) |
| HRZES | 3 (0.6) |
| R | 10 (1.9), all MDR |
| S | 23 (4.4) |
| S | 8 (1.5) |
| HS | 10 (1.9) |
| MDR | 5 (1.0) |
| Z | 11 (2.1), 5 MDR |
| E | 5 (1.0), all MDR |
E: ethambutol; H: isoniazid; MDR: multi-antituberculosis drug resistance. R: rifampicin; S: streptomycin; Z: pyrazinamide. The different combinations of resistance to H, R and other drugs correspond to the 10 MDR cases.
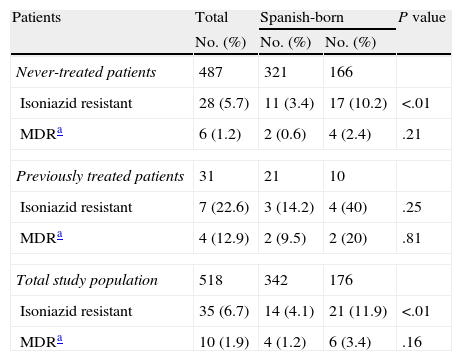
Isoniazid Resistance and Multi-resistance According to the Type of Resistance and Origin (Spanish or Foreign) of the RETUBES Study Patients (No.=518).
| Patients | Total | Spanish-born | P value | |
| No. (%) | No. (%) | No. (%) | ||
| Never-treated patients | 487 | 321 | 166 | |
| Isoniazid resistant | 28 (5.7) | 11 (3.4) | 17 (10.2) | <.01 |
| MDRa | 6 (1.2) | 2 (0.6) | 4 (2.4) | .21 |
| Previously treated patients | 31 | 21 | 10 | |
| Isoniazid resistant | 7 (22.6) | 3 (14.2) | 4 (40) | .25 |
| MDRa | 4 (12.9) | 2 (9.5) | 2 (20) | .81 |
| Total study population | 518 | 342 | 176 | |
| Isoniazid resistant | 35 (6.7) | 14 (4.1) | 21 (11.9) | <.01 |
| MDRa | 10 (1.9) | 4 (1.2) | 6 (3.4) | .16 |
MDR: multidrug resistant.
Among the 518 cases with a known history of previous anti-TB treatment, 487 (94.0%) had never been treated (321 Spanish-born and 166 foreign-born patients in a similar percentage, 94%), while 31 (6.1%) cases had been previously treated (21 Spanish-born and 10 foreign-born patients). Of the 35 H-resistant patients, 28/487 (5.7%) had not been previously treated, while 7/31 (22.6%) had received treatment. Of the 10 patients with MDR-TB, 6/487 (1.2%) had never been previously treated, while 4/31 (12.9%) had received treatment. Eleven of the 28 never-treated patients with DR-TB-H were Spanish-born (3.4%) and 17 (10.2%) were foreign-born (Table 3). In total, 70% of study patients commenced TB treatment with 4 drugs (HRZE) and 22% with a 3-drug regimen (HRZ) (Table 1).
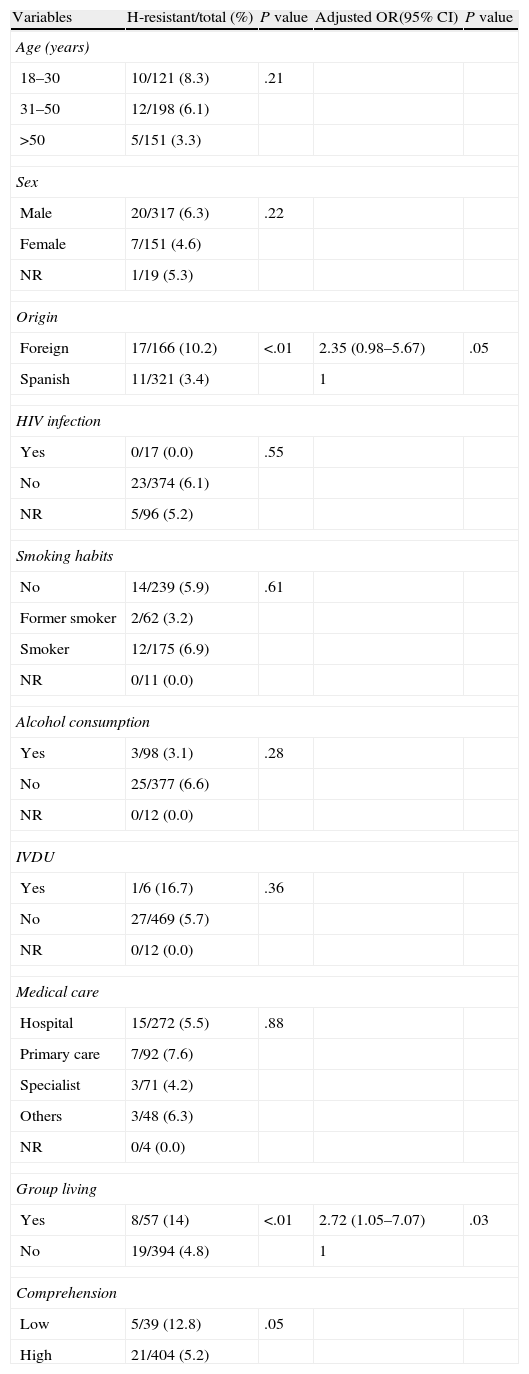
Risk factors associated with primary DR-TB-H were immigrant status and group cohabitation (Table 4). Age under 50 years was also a factor associated with acquired DR-TB-H (Table 5).
Factors Predicting Isoniazid Resistance in Never-treated Patients (No.=487). Univariate and Multivariate Analyses.
| Variables | H-resistant/total (%) | P value | Adjusted OR(95% CI) | P value |
| Age (years) | ||||
| 18–30 | 10/121 (8.3) | .21 | ||
| 31–50 | 12/198 (6.1) | |||
| >50 | 5/151 (3.3) | |||
| Sex | ||||
| Male | 20/317 (6.3) | .22 | ||
| Female | 7/151 (4.6) | |||
| NR | 1/19 (5.3) | |||
| Origin | ||||
| Foreign | 17/166 (10.2) | <.01 | 2.35 (0.98–5.67) | .05 |
| Spanish | 11/321 (3.4) | 1 | ||
| HIV infection | ||||
| Yes | 0/17 (0.0) | .55 | ||
| No | 23/374 (6.1) | |||
| NR | 5/96 (5.2) | |||
| Smoking habits | ||||
| No | 14/239 (5.9) | .61 | ||
| Former smoker | 2/62 (3.2) | |||
| Smoker | 12/175 (6.9) | |||
| NR | 0/11 (0.0) | |||
| Alcohol consumption | ||||
| Yes | 3/98 (3.1) | .28 | ||
| No | 25/377 (6.6) | |||
| NR | 0/12 (0.0) | |||
| IVDU | ||||
| Yes | 1/6 (16.7) | .36 | ||
| No | 27/469 (5.7) | |||
| NR | 0/12 (0.0) | |||
| Medical care | ||||
| Hospital | 15/272 (5.5) | .88 | ||
| Primary care | 7/92 (7.6) | |||
| Specialist | 3/71 (4.2) | |||
| Others | 3/48 (6.3) | |||
| NR | 0/4 (0.0) | |||
| Group living | ||||
| Yes | 8/57 (14) | <.01 | 2.72 (1.05–7.07) | .03 |
| No | 19/394 (4.8) | 1 | ||
| Comprehension | ||||
| Low | 5/39 (12.8) | .05 | ||
| High | 21/404 (5.2) | |||
H: isoniazid; CI: confidence interval; NR: not recorded; OR: odds ratio; IVDU: intravenous drug user; HIV: human immunodeficiency virus.
The total numbers for age, family and comprehension do not total 28, because data from 1 or 2 patients were lost.
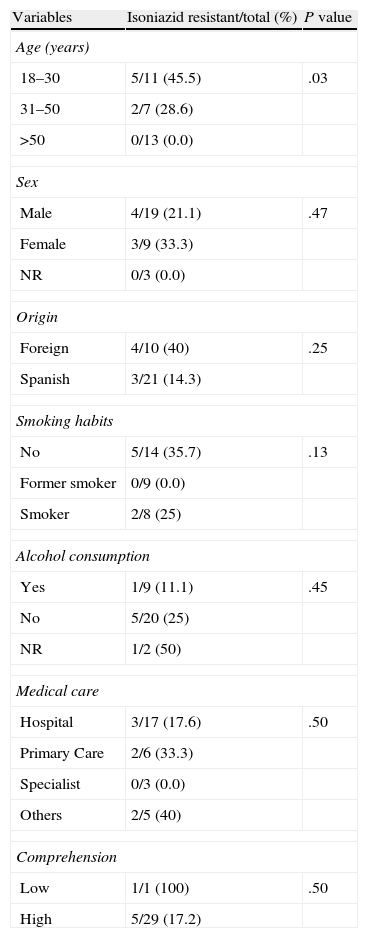
Factors Predicting Isoniazid Resistance in Previously Treated Patients (No.=31). Univariate Analysis.
| Variables | Isoniazid resistant/total (%) | P value |
| Age (years) | ||
| 18–30 | 5/11 (45.5) | .03 |
| 31–50 | 2/7 (28.6) | |
| >50 | 0/13 (0.0) | |
| Sex | ||
| Male | 4/19 (21.1) | .47 |
| Female | 3/9 (33.3) | |
| NR | 0/3 (0.0) | |
| Origin | ||
| Foreign | 4/10 (40) | .25 |
| Spanish | 3/21 (14.3) | |
| Smoking habits | ||
| No | 5/14 (35.7) | .13 |
| Former smoker | 0/9 (0.0) | |
| Smoker | 2/8 (25) | |
| Alcohol consumption | ||
| Yes | 1/9 (11.1) | .45 |
| No | 5/20 (25) | |
| NR | 1/2 (50) | |
| Medical care | ||
| Hospital | 3/17 (17.6) | .50 |
| Primary Care | 2/6 (33.3) | |
| Specialist | 0/3 (0.0) | |
| Others | 2/5 (40) | |
| Comprehension | ||
| Low | 1/1 (100) | .50 |
| High | 5/29 (17.2) | |
NR: not recorded.
Excluding variables with P>.5.
Adequate control of TB requires regularly updated information on the prevalence of MT-resistance to anti-TB drugs. In 2007, the Spanish National Health System, at the request of the SEPAR Area for Tuberculosis and Respiratory Infections, created the Plan for the Prevention and Control of Tuberculosis in Spain, in which it recommended the systematic study of TB using DST.9 However, the results published to date by the National Epidemiological Surveillance Network (RENAVE) do not include these data.10,11 This is the first prospective study on DR-TB conducted in Spain by a scientific society using systematic DST in a large patient cohort.
Our study found an acceptable prevalence of MT resistance to first-line drugs, especially in the indigenous population (Tables 2 and 4), although higher than that described in previous studies.3,8 This is probably due to the migratory phenomenon observed in Spain in recent years. We noted that all cases of R-resistance were MDR-TB, XDR-TB was absent (in contrast to a previous study12), and we were able to detect risk factors for DR-TB-H.
The DR-TB-H rate in our study was higher than that found in Portugal, similar to rates in France and Denmark, and lower than rates found in Italy and other Central European and Nordic countries with a higher proportion of foreign-born patients.13–15 The increase in DR-TB-H, which has been associated with TB transmission in the foreign-born population,16 may spread infection by H-resistant strains, especially among younger subjects.17
The rates of DR-TB-H and MDR-TB in never-treated cases accurately reflect the recent transmission of TB, with H-resistance considered to be the first step toward the development of MDR-TB and XDR-TB.3,18 Primary DR-TB-H, i.e. DR-TB-H diagnosed before commencing treatment of a new patient with no history of previous anti-TB therapy,19,20 may include primary resistance or hidden acquired resistance, but is usually transmitted.18
The actual rate of primary DR-TB-H in Spain has been hitherto unknown, while the rate of primary MDR-TB was estimated at 2%,21 exceeding the previous estimation of 0.2%.3 The fact that the primary DR-TB-H rate in our study is higher than that reported for Spain by international organizations3,4 and in a previous study8 is cause for concern. Even so, the primary DR-TB-H and MDR-TB rates observed are lower than the mean rates for the European Community (EC) in 2010 (7.8% and 2.6%, respectively), similar to those described for Great Britain and Portugal, and lower than those of other Central European and Nordic countries with a higher proportion of foreign-born patients.22 In an excellent review of previous studies, rates in Spain were found to be much higher than those reported for never-treated patients, with a mean rate of 3.0% for DR-TB-H, with large variability (0%–5.1%), and 0.37% for MDR-TB.23 In subsequent local studies, the rate of primary DR-TB-H ranged from 1.9% to 4.4%, reaching 5.3% in a region with high immigration,24–27 while the primary MDR-TB rate did not exceed 1.4%.25,26
Resistance to anti-TB drugs in previously treated cases is usually labeled as acquired resistance, although it may also include patients initially infected or reinfected by a resistant strain.3,18 Our rates of acquired DR-TB-H (22.6%) and acquired MDR-TB (12.9%) are similar to those of another national study,8 lower than the mean for the EC, Romania, Bulgaria and Sweden, but higher than those of other countries.28
The likelihood of generating a resistant strain during TB treatment is greatest during the initial intensive treatment phase, when the bacterial population is highest.29 In total, 70% of our patients commenced treatment with a 4-drug regimen (HRZE), higher that the rate reported in a previous study,12 while 22% did so with a 3-drug regimen (HRZ), some of these smear-negative, with positive culture and known DST results. In new patients with no previous disease, the 4-drug regimen should be commenced and maintained until the DST results are known. If such testing is not available, the likelihood of DR-TB must be estimated in order to prevent the development of resistant strains,30–33 as the incidence of failures, drop-outs, acquired resistance, and even MDR-TB is higher among cases with unidentified primary DR-TB, especially R-resistant and to a lesser extent H-resistant, that have been treated with a standard (particularly 3-drug) regimen.19,29,33
In our study, never-treated patients who were of foreign origin or who cohabited in a group had a higher risk of presenting primary resistance. Being foreign-born is a well-known risk factor for DR-TB and MDR-TB.20,34–36 Foreign migrants are generally economic immigrants from countries with limited control programs, while group living, which tends to overlap with other conditions,20 can facilitate TB or a latent tuberculosis infection (LTBI) by H-resistant MT, in which standard treatment of LTBI will be ineffective.37
Our study patients are considered to be a relatively representative sample of the Spanish TB population, albeit with fewer patients co-infected with HIV.10,11 We believe that our proportion of never-treated patients (94%), which is similar to national figures (92.8%) and to previous studies,7,8,10,11,26 could be due to satisfactory treatment outcomes in around 90% of cases7,12,26 (higher than both national figures 11 and the mean reported in the EC and several European countries6,13). We attribute this to both patient follow-up in the public health system, with few losses and drop-outs,7,12 and the availability of fixed-dose combined drugs for the treatment of TB, and also to the proportion of foreign-born patients included in our study (34.1%), which is lower than in other European countries.10–13
Our study has several limitations. The microbiological study was not centralized, although fully accepted and recognized methods were used. The MT isolation procedure was performed in each hospital or reference center by sample culture grown on solid or liquid medium, confirmed by culture grown on solid medium, and MT was identified by gene probe or using an automated method. We did not examine all DST methods in reference laboratories, but according to routine diagnostic procedures used in the national healthcare network, testing was performed using recognized methods for reliably detecting resistance to H and R,38 most using the BACTEC™ MGIT 960 system and the rest using the proportions method. Other limitations are that 4 Spanish regions did not participate in the study, nor was the prison population included, although in our opinion, this did not distort sample representativeness.10
In conclusion, the results of this prospective study show a slight increase in resistance to anti-TB drugs in Spain, which could be due to mass immigration in recent years. The rates of H-resistance and MDR-TB in never-treated patients indicate the need for systematic surveillance. Finally, we conclude that never-treated patients from other countries and those who live in a group have a higher risk of being H-resistant.
FundingThe study was financed by Grant number 886 of the call for SEPAR Research Grants 2008.
Conflict of InterestsThe authors do not have any conflicts of interest.
N. Altet (Unidad Prevención y Control Tuberculosis, Barcelona); F. Álvarez-Navascués (H. San Agustín, Avilés, Asturias); M. Barrón (H. San Millán-San Pedro, Logroño); R. Blanquer (H. Dr. Peset, Valencia); A. Bustamante (H. Sierrallana, Torrelavega); J.L. Calpe (H. La Marina Baixa, Villajoyosa); J.A. Caminero (Complejo Hospitalario Dr. Negrín, Las Palmas de Gran Canaria); F. Cañas (H. Insular de Gran Canaria, Las Palmas de Gran Canaria); M. Casals (Agencia de Salud Pública de Barcelona, Barcelona); X. Casas (H. Sant Boi, Barcelona); J.A. Caylà (Agencia de Salud Pública de Barcelona, Barcelona); M.L. de Souza (Unidad Prevención y Control Tuberculosis, Barcelona); J. Gallardo (H. Universitario de Guadalajara, Guadalajara); M. Gallego (Corporación Sanitaria Parc Taulí, Sabadell); F.J. García (H. Universitario de la Princesa, Madrid); J.M. García-García (H. San Agustín, Avilés, Asturias); J.A. Gullón (Hospital Universitario de Canarias, La Laguna); M.A. Jiménez (Unidad Prevención y Control Tuberculosis, Barcelona); T. Lloret (H. General Universitario de Valencia, Valencia); M. Marín (H. General de Castellón, Castellón); J.F. Medina (H Universitario Virgen del Rocío, Sevilla); C. Milà (Unidad Prevención y Control Tuberculosis, Barcelona); I. Mir (H. Son Llàtzer, Palma de Mallorca); V. Moreno (H. Carlos III, Madrid); T. Pascual (H. de Cabueñes, Gijón); A. Penas (Complejo Hospitalario Xeral-Calde, Lugo); T. Rodrigo (Fundación Respira de SEPAR); J. Ruiz-Manzano (H. Universitario Germans Trías i Pujol, Badalona); J. Sala (H. Universitario Joan XXIII, Tarragona); P. Sánchez (H. del Mar, Barcelona); F. Sanz (H. General Universitario de Valencia, Valencia); E. Valencia (H. Carlos III, Madrid); A. Vargas (H. Universitario Puerto Real, Cádiz); R. Vidal (H. Vall D’Hebrón, Barcelona); M.A. Villanueva (H. San Agustín, Avilés, Asturias).
The names of the components of the Working Group of the Integrated Programme for Research in Tuberculosis in Spain are listed in Annex.
Please cite this article as: Blanquer R, Rodrigo T, Casals M, Ruiz Manzano J, García-García JM, Calpe JL, et al. Resistencia a fármacos antituberculosos de primera línea en España durante 2010–2011. Estudio RETUBES. Arch Bronconeumol. 2015;51:24–30.