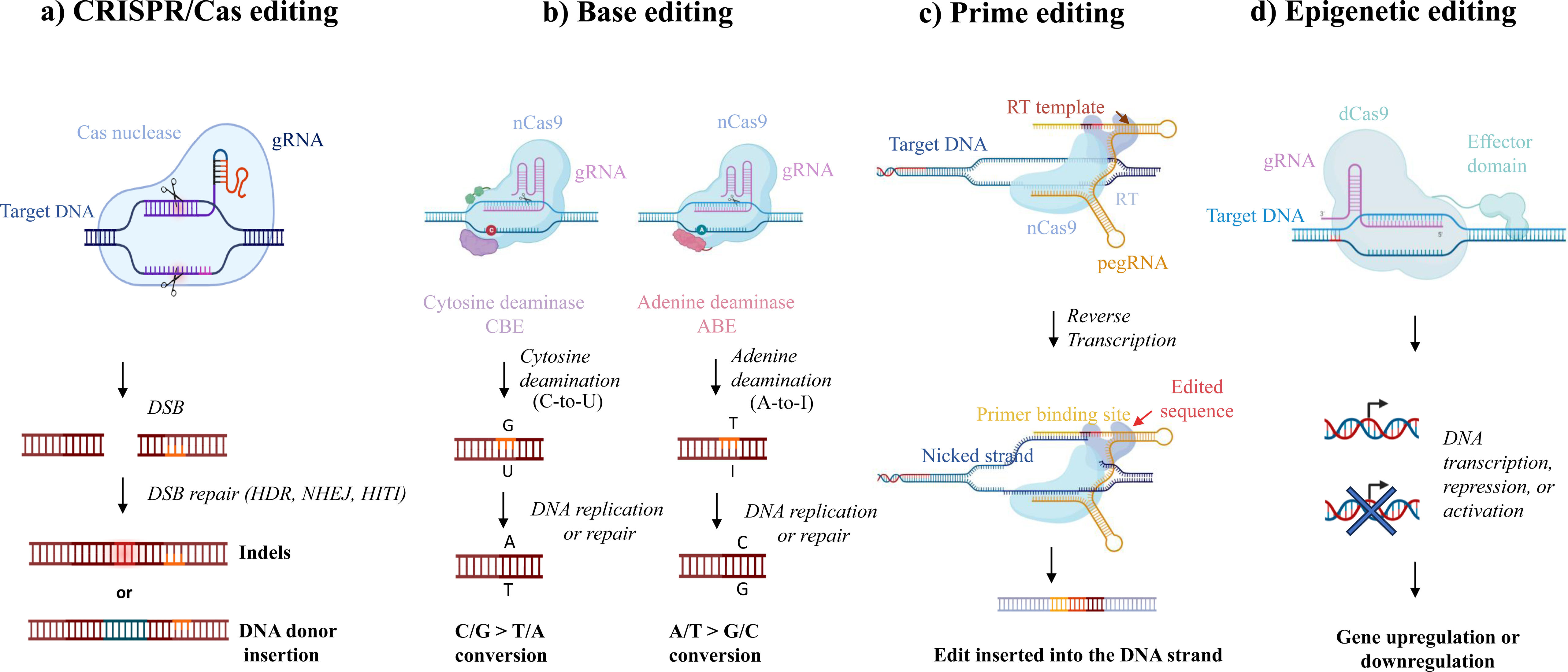
Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR), sometimes referred to as CRISPR-associated protein 9 (Cas9) endonuclease, is a method used for gene or genome editing that has revolutionized the field of biomedicine. Researchers can use this technology to modify genes by adding, deleting, or altering portions of deoxyribonucleic acid (DNA). CRISPR/Cas9 has two main components: (1) a single guide ribonucleic acid (gRNA) molecule that leads the Cas9 protein to the targeted section of the DNA sequence to be modified and (2) the Cas9 protein itself which can precisely cut the DNA at specific locations, thus acting as molecular “scissors”. The consequent double-strand break (DSB) can result in a gene knockout or, after the targeted insertion of a specific DNA sequence into a specific locus, a gene knockin.1 Unlike traditional CRISPR/Cas9, base editing, prime editing, and epigenetic editing are the next generation of CRISPR-based gene methods that do not involve DSB (Fig. 1). In doing so, the risk of off-target genome alterations as a potential result of DNA repair (e.g., unpredictable rearrangements, translocations, indel byproducts) is minimized.
CRISPR-based editing systems. The main CRISPR editing systems include: (a) classical CRISPR/Cas editing, in which a Cas nuclease cuts the DNA, creating a double-strand break (DSB), after which native repair mechanisms can introduce and correct the functional genetic material initially absent via homology-directed repair (HDR), non-homologous end joining (NHEJ), or homology-independent targeted integration (HITI); (b) base editing, in which changes to individual DNA base pairs are induced by the utilization of an engineered form of Cas9 (Cas9 nickase); adenine base editors (ABE) or cytosine base editors (CBE) are the two specific types; (c) prime editing, which involves a catalytically impaired Cas9 nickase (nCas9) fused with an engineered reverse transcriptase (RT) and complexed with a prime editing guide RNA (pegRNA); and (d) epigenetic editing, which involves altering the expression of a specific gene without modifying its underlying DNA sequence. This can be achieved through processes such as DNA methylation and histone acetylation. To facilitate this, a non-cutting variant of the Cas9 enzyme, referred to as “dead” Cas9 (dCas9), is utilized. dCas9 can be combined with various effector domains that can modulate gene expression at specific locations in an epigenetic manner.
The application of CRISPR technology to the research of conditions with important genetic backgrounds (e.g., cystic fibrosis [CF]) or where genetic factors may play a role (e.g., lung cancer), is particularly advantageous given the possibility of faithfully creating disease models, identifying responsible trigger or protective genes, offering an early diagnosis, and providing a gene-based therapy.2
Knowledge of the status of oncogenic driver genetic variants is paramount for the management of advanced non-small cell lung cancer (NSCLC). Numerous gene alterations have been reported to affect therapeutic selection in patients with NSCLC. The main applications of CRISPR in lung cancer research are disease modelling, identification of novel oncogenic drivers, tumour suppressor genes (TSG), and genes that may predict tumour responses, as well as gene-based therapy.2 In lung cancer cells or animal models, CRISPR/Cas9 can be used to suppress or produce an activating genetic variation in known TSGs or oncogenes, respectively. Genome-wide CRISPR/Cas9 screening has been used for gene identification. They consist of a library of gRNAs designed to target multiple genes within the entire genome to determine their function and the phenotypic consequences of their modification. For instance, genome-wide CRISPR/Cas9 screens on mutants NSCLC cell lines may identify genes whose deletion/loss increases the efficacy of certain targeted therapies.3 CRISPR technology has been able to define TSG that may be critical for the development of lung cancer.4 Also, through the use of CRISPR libraries it has been reported that mucin 21 (MUC21) protects cancer cells from natural killer (NK)-cytotoxicity and T-cell mediated killing.5 Therefore, targeting MUC21 may result in an enhancement of the effectiveness of immunotherapy in the future. Another application, rather unexplored, is the usage of biosensors integrated with built-in CRISPR nucleases (e.g., Cas9, Cas12a, Cas 13a, Cas14a) to detect specific cancer-related DNA or RNA sequences in bodily fluids, which may represent a quick, highly sensitive and specific method for diagnosing cancer.6
Only a single human phase I clinical trial has demonstrated that the clinical application of CRISPR/Cas9 programmed cell death protein 1 (PD-1) edited T-cell therapy is safe and feasible.7 In that trial, 12 NSCLC patients received a transfusion of autologous T cells with PD-1 knocked out ex vivovia CRISPR/Cas9 (gene-edited T cells). There were no major adverse events higher than grade 2, and the incidence of off-target events was low. However, the objective clinical response was limited, indicating that more effective CRISPR-based cell therapies should be investigated. There is another phase I/II trial in the process of recruitment, in which the intracellular immune checkpoint cytokine-induced SH2 protein (CISH) of tumour-infiltrating lymphocytes will be inactivated using CRISPR gene editing (clinical trial NCT05566223).8 Presently, the advancement of genetically engineered T-cell therapy for NSCLC lags considerably behind that of other tumours.
CF is an autosomal recessive disease caused by genetic variations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene that encodes a chloride channel. Chloride channel dysfunction leads to abnormally thick secretions, which are difficult to clear. Although over 2000 genetic variants have been described, the most common genetic variant resulting in disease is deltaF508 (F508del), defined as a deletion of phenylalanine at position 508 of the CFTR gene.9 Depending on the genetic variant in an individual patient, different CFTR modulator drugs can be considered for chronic treatment. In a simplified form, CFTR modulators either improve the intracellular processing of CFTR protein to the apical membrane of the epithelial cell (“correctors” such as tezacaftor), the function of the CFTR expressed at the cell membrane (“potentiators” such as ivacaftor), or both (e.g., elexacaftor).9 However, the use of highly effective modulator therapy (e.g., elexacaftor/tezacaftor/ivacaftor combination) does not always improve patients sufficiently, or patients may not be eligible for these medications because of their genotype.10 Newer gene-based therapies aimed at fixing or replacing the malfunctioning CFTR gene are on the horizon. And, according to a recent survey of CF researchers, CRISPR/Ca9 was considered by far the most promising therapy for this purpose in the coming years.11
CRISPR-based editing approaches to correct CFTR genetic variations have been investigated in cultured airway basal cells, induced pluripotent stem cells (iPSC), advanced cellular models (e.g., organoid cultures derived from patients with CF), and several animal models (e.g., rodents, rabbits, sheep, lambs, pigs, and ferrets). Schwank et al. first reported the use of the CRISPR/Cas9 genome editing system to correct the CFTR locus by homologous recombination in cultured intestinal stem cells of CF patients.12 Firth et al. described the CFTR gene correction of iPSC obtained from the skin fibroblasts of a patient with F5089del using CRISPR.13 Lung epithelial cells subsequently derived from corrected iPSC demonstrated CFTR recovered function. More recently, Li et al. demonstrated the correction of the CFTR nonsense genetic variant W1282X in iPSCs from CF patients using prime editing machinery.14 This correction with the subsequent restoration of CFTR function can also be achieved through adenine base editing.15 Epigenetic editing has been employed in a limited number of studies. In one such investigation, researchers utilized dCas9, which was fused with VP64-p65-Rta, to activate the expression of CFTR in cultured human nasal epithelial cells derived from CF patients.16 Although editing of CFTR mutants is feasible with the use of cell lines, organoids, or animal models, a major challenge is the delivery of editing tools into target lung cells (i.e., in vivo CFTR correction). Since lung epithelial cells regenerate periodically, airway basal progenitor cells need to be targeted to achieve permanent correction. Several delivery systems, including nanoparticles, viral vectors, and engineered virus-like particles, have been proposed. Basically, it can be considered the transplantation through the tracheobronchial tree of ex vivo edited autologous cells, or the in vivo editing of basal or epithelial cells. In vivo gene editing refers to the process by which genetic modifications are made directly to the affected tissue, specifically the lungs in the case of CF and lung cancer. This method has several applications, including disease modelling, identification of disease-causing and protective genes, and gene therapy. In contrast, ex vivo gene editing involves genetic modifications to cells that have been removed from the patient or animal and grown in a laboratory setting. The main applications of ex vivo gene editing include disease modelling, identification of disease-causing and protective genes, early diagnosis, and therapy.
Despite being a powerful tool for gene editing, CRISPR technology is still in its infancy, as far as human gene therapy is concerned. Current obstacles and challenges in the clinical application of CRISPR include the low efficiency of gene editing in achieving the desired DNA changes, particularly for gene correction via homology-directed repair (HDR); the lack of robust delivery methods; the potential for germline modifications, known as genotoxicity, which may increase the risk of malignant transformation; the phenomenon of genetic mosaicism, which occurs when CRISPR is employed in embryos, as the system can continue to target and cleave genes at different stages of embryonic development; the possibility of an immune reaction to CRISPR components and delivery vehicles, as Cas9 is derived from a bacterium that causes many common infections in humans; the need to implement a long-term expression or repeated dosing; and mutational variation among patients with the same disease. In this case, customizing CRISPR therapy for each individual presents both economic and timely challenges for scaling up pharmaceutical manufacturing.17 From the ethical point of view, since CRISPR involves changing the human genome, in most cases irreversibly, concerns have been expressed. The creation of inheritable changes in human germline cells or embryos remains highly controversial as it could result in permanent changes passed down through generations. Furthermore, undesired off-target modifications can be produced and propagated owing to limitations in the technique. Therefore, it is essential to establish ethical guidelines to ensure the prevalence of advantages and mitigate potential risks. Ongoing genomic research may help overcome these obstacles and make gene therapy a viable option in the near future.
FundingThis article had no funding sources.
Conflicts of InterestsThe authors declare no conflicts of interest.