The aim of this review is to assist pulmonologists in the management of diseases involving both the upper and lower respiratory tract that are linked by a common, interrelated epidemiology, clinical signs and symptoms, and inflammatory mechanism , asthma, in particular.
The document discusses the definitions of the various sinonasal phenotypes associated with asthma: allergic and non-allergic rhinitis and chronic rhinosinusitis with or without nasal polyps. Diagnostic criteria and severity levels are also listed.
Particular attention has been given to the 2 main syndromes associated with asthma: (i) allergic rhinitis, the most common, and (ii) chronic rhinosinusitis with nasal polyps, the disease most closely associated with severe asthma.
To summarize, the upper respiratory tract should always be evaluated in order to achieve a single diagnosis and comprehensive treatment of the “united airway”.
El objetivo de esta revisión es facilitar al neumólogo el manejo de las enfermedades de la vía respiratoria superior ligadas a la vía respiratoria inferior, especialmente al asma, unidas por una epidemiología, una clínica y un mecanismo inflamatorio comunes e interrelacionados.
El documento recoge las definiciones de los diferentes fenotipos nasosinusales ligados al asma: rinitis alérgica o no alérgica y rinosinusitis crónica con o sin pólipos nasales. Asimismo se recogen los criterios diagnósticos y su nivel de gravedad.
Se dedica especial atención a los dos síndromes principales asociados al asma: 1) rinitis alérgica, la patología más frecuente, y 2) rinosinusitis crónica con pólipos nasales, la patología más ligada al asma grave.
En síntesis, en el manejo del asma debe valorarse siempre la vía respiratoria superior con la finalidad de un diagnóstico unificado y un tratamiento integral de la vía respiratoria única.
The respiratory tract, from the nose to the bronchi, forms a functional anatomical unit. Abundant scientific evidence shows that asthma and rhinitis/rhinosinusitis share a common, interrelated epidemiology, clinical picture, and inflammatory mechanism. All this evidence has led to the emergence of the “united airways” concept, establishing the need for an integrated approach to the diagnosis and treatment of airway diseases.1–3
This document discusses the definitions, severity levels, and therapeutic consensus documents, particularly for allergic and non-allergic rhinitis and chronic rhinosinusitis with or without nasal polyps in asthma.1,2 The ultimate goal of this review is to guide pulmonologists in the management of diseases of the upper respiratory tract, especially in asthma patients. To this end, we have integrated the diagnostic and therapeutic aspects contained in national and international consensus documents.
MethodologyThis document has been drawn up using the following methodology.
- 1
A MEDLINE literature search was performed, using the key words describing the patient population: “united airways”, “asthma and rhinitis”, “asthma and chronic rhinosinusitis”, “asthma and allergic rhinitis” and “asthma and nasal polyps”, along with key words describing the intervention: “treatment of allergic rhinitis”, “treatment of chronic rhinosinusitis” and “treatment of nasal polyposis”. Studies that met the following criteria were included: (a) related with allergic rhinitis and chronic rhinosinusitis in asthmatic patients; (b) controlled, randomized, or observational studies; and (c) published in English or Spanish.
- 2
These SEPAR guidelines were drawn up on the basis of all the studies included, while the recommendations were made according to the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) method.4 Scientific evidence and therapeutic recommendations are based on the international consensus for rhinitis, Allergic Rhinitis and its Impact on Asthma (ARIA),1 which also uses the GRADE method4 as a basis for selecting criteria for scientific evidence. For chronic rhinosinusitis (CRS), we used the 2012 European position paper on rhinosinusitis and nasal polyps (EPOS 2012),2 based on the criteria for scientific evidence described by Shekelle et al.,5 and the Spanish guidelines on nasal polyposis, POLINA,6 and on asthma, rhinitis, and nasal polyposis (Spanish Guidelines on the Management of Asthma [GEMA] 4.1).7 All these documents have been reviewed by the authors and international external reviewers.
- 3
All members of the group (pulmonologists specializing in asthma, allergologists, and ENT specialists) reviewed the final document, which was approved by all members of the group.
Inflammation of the nasal mucosa is characterized by the following symptoms: nasal obstruction, rhinorrhea, sneezing and nasal pruritus/itching. It is often accompanied by ocular symptoms, such as pruritus and watery eyes.1
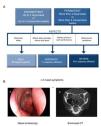
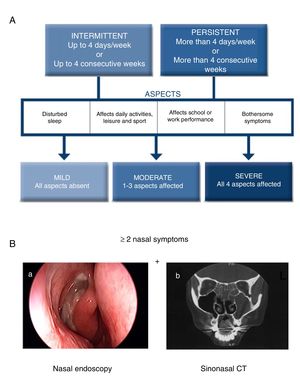
The current ARIA guidelines1 support a new classification that is more useful in clinical practice, defined by the duration of symptoms (intermittent, persistent) and the severity of the disease (mild, moderate, severe) (Fig. 1A). Two main phenotypes have been identified: (a) allergic rhinitis (AR), mediated by IgE after exposure to the allergen and skin prick tests and/or blood tests positive for inhaled allergens, and (b) non-allergic rhinitis (NAR), with negative allergy tests. The latter is less well-defined entity with a etiopathogenesis that can be inflammatory, hormonal or neurogenic.
Diagnostic criteria for allergic rhinitis and chronic rhinosinusitis with nasal polyps (CRSwNP). (A) Classification of allergic rhinitis defined by duration and severity, according to the ARIA guidelines (adapted from Valero et al.27). (B) Chronic rhinosinusitis is defined by the presence of at least two sinonasal symptoms, one of which must be either nasal obstruction/congestion/blockage or rhinorrhea (anterior/posterior), along with facial pain/pressure or reduction/loss of sense of smell lasting longer than 12 weeks, in addition to any of the following findings: endoscopic signs of nasal polyps, mucopurulent rhinorrhea of the middle meatus, edema/obstruction of the mucosa (middle meatus) and/or changes in the CT scan of the paranasal sinuses. (B) (a) Endoscopic vision of nasal polyp in the middle meatus, and (b) sinonasal CT image with involvement of the left maxillary sinus and bilateral ethmoid sinuses in a patient with CRS (2).
A new entity, local allergic rhinitis, mediated by IgE and characterized by symptoms of allergic rhinitis but with negative skin and blood tests, has recently been described.8 Diagnosis is based on a positive reaction to nasal provocation with the clinically relevant nasal allergen.
Chronic RhinosinusitisInflammation of the nasal mucosa and paranasal sinuses lasting over 12 weeks that manifests with 2 or more of the following symptoms, one of which must be congestion/obstruction/blocked nose and/or anterior/posterior rhinorrhea with or without the presence of pain/facial pressure and/or reduction/loss of sense of smell, and confirmed on nasal endoscopy and/or sinonasal computed tomography (Fig. 1B).2 There are two main phenotypes of chronic rhinosinusitis: chronic rhinosinusitis with nasal polyps (CRSwNP) and without nasal polyps (CRSsNP).
CRS is considered a heterogeneous group of diseases with different etiologies and pathophysiological mechanisms, within which nasal polyposis constitutes a differentiated phenotype.2,6
EpidemiologyAllergic and Non-allergic RhinitisAR is the most common type of non-infectious rhinitis. It represents a global health problem which affects 21.5% of the Spanish population.9 Most patients with allergic or non-allergic asthma have concomitant rhinitis (nasal symptoms). In Spain, recent studies in pulmonology,10 allergology,11 and primary care12,13 have shown a high prevalence of mostly allergic rhinitis (71%–90%), in subjects with asthma. In other studies conducted in Spain and Portugal, between 37% and 49% of patients with allergic rhinitis, respectively, had asthma.14,15 Rhinitis, whether allergic or non-allergic, usually precedes the onset of asthma3,16 and is considered a risk factor for the development of asthma.
Chronic Rhinosinusitis With Nasal PolypsCRS is a highly prevalent disease that affects 11% of the adult population in Europe.17,18 CRSwNP is a CRS phenotype that occurs in the general population with a prevalence of around 2%–4%.16 It is clearly more predominant among men (2 to 1) and has a great impact on quality of life.19
Recent data20 show that almost 20% of patients with asthma have CRSwNP, and this rate increases among patients with non-atopic disease. A group of asthma patients with CRSwNP also are intolerant to aspirin and non-steroidal anti-inflammatory drugs (NSAIDs), the so-called Widal or Samter triad that is now known as NSAID-exacerbated respiratory disease (NERD). This entity is the most serious and recurrent clinical form of nasal polyposis associated with severe asthma.21–23
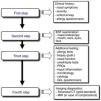
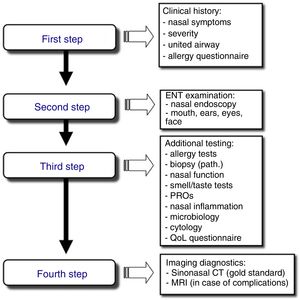
Stepwise Diagnosis in the Management of Sinonasal Disorders (Fig. 2)First Step: Clinical Expression of the Sinonasal DiseaseNasal symptoms, described previously and based on the clinical history, guide the diagnosis and classification of rhinitis and CRS by duration and severity (Fig. 1A, B).24,25
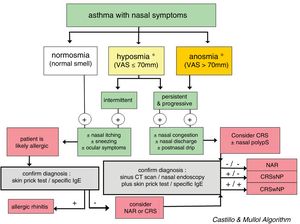
In CRS, the symptom that best predicts the endoscopic or radiological diagnosis is an altered sense of smell, especially when it coexists with another nasal symptom (Fig. 3).26 Given the nonspecific nature of the symptoms (a high rate of false positives), nasal endoscopy and/or sinonasal CT is required to confirm the diagnosis.2
Diagnostic algorithm based on the loss of smell to discriminate between rhinitis and chronic rhinosinusitis in asthmatic patients. The figure shows the diagnostic algorithm for asthma patients with nasal symptoms to discriminate between rhinitis, with or without allergy, and chronic rhinosinusitis, with or without nasal polyps. CRSsNP: chronic rhinosinusitis without nasal polyps; CRSwNP: chronic rhinosinusitis with nasal polyps; NAR: non-allergic rhinitis; VAS: visual analog scale. *In case of hyposmia/anosmia, other causes, such as a head trauma (e.g., accident) or a viral syndrome (e.g., common cold or flu), should always be ruled out first.
According to ARIA, the severity level of AR can be assessed by evaluating the impact on 4 aspects of quality of life (Fig. 1A).1,27 According to EPOS, CRS severity is evaluated with a visual analog scale (VAS, from 0 to 10cm) according to the patient's response to the question “How troublesome are your symptoms of rhinosinusitis?”. Severity is classified as mild (EVA>0–3cm), moderate (EVA>3–7cm) or severe (EVA>7cm).2
Control of AR can be evaluated by using validated questionnaires.28–30
Second Step: ENT ExaminationRhinoscopy, and preferably nasal endoscopy, are essential examinations, given their high diagnostic yield. Both rigid and flexible nasal endoscopes can be used. This examination should ideally be performed in all patients to optimize the differential diagnosis.
Third Step: Additional StudiesAllergy tests must be performed to diagnose respiratory allergy, the most common cause of chronic nasal symptoms in early-onset asthma. Clinical guidelines recommend skin prick tests or the determination of specific IgE in blood.31 Second-line tests include: (a) nasal function and permeability tests, using anterior nasal rhinomanometry, acoustic rhinometry, or peak nasal inspiratory flow (PNIF); (b) smell tests, such as BAST-24, a subjective, well validated and culturally adapted smell test developed in Spain, or the use of a VAS (0–10cm), which facilitates the quick and easy evaluation of olfactory loss in daily clinical practice; (c) nasal cytology, which is a simple test with a dubious yield; and (d) nasal provocation tests, both specific tests and reference tests for the etiological diagnosis of allergy, especially occupational allergy and local allergic rhinitis, and nonspecific provocation (cold), which are of interest in the study of the nasal hyperreactivity. There are standardized consensuses for the conduct of these tests.32,33
Fourth Step: Diagnostic ImagingSinonasal computed tomography (CT) is considered the gold standard, although magnetic resonance imaging (MRI) can be used if complications are suspected. Standard X-ray is not indicated due to its low yield.
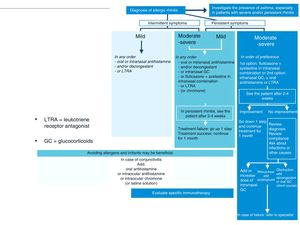
Treatment of Allergic Rhinitis in Patients With AsthmaAntihistamines and intranasal corticosteroids are the cornerstone of AR treatment. These drugs are not indicated for the treatment of asthma, although a recent meta-analysis found that intranasal corticosteroids34 have a beneficial effect in both the control and prevention of asthma exacerbations (Fig. 4, Tables 1 and 2).
Stepwise treatment algorithm for allergic rhinitis. This algorithm establishes treatment according to the duration of symptoms (intermittent or persistent) and disease severity (mild, moderate-severe). GC: glucocorticoids; LTRA: leukotriene receptor antagonists.
Scientific Evidence and Recommendations for the Treatment of Allergic Rhinitis and Asthma in Patients With Rhinitis and Asthma According to GRADE.
| Treatments | Allergic Rhinitis | Asthma in Allergic Rhinitis+Asthma |
|---|---|---|
| Oral anti-H1 (2nd generation)a | Yes/Strong/Low (1C) | No/Weak/Very Low (2D) |
| Intranasal anti-H1a | Yes/Weak/Low (2C) | No data |
| Anti-leukotrienesa | Yes/Weak/High (2A) | Yes/Weak/Moderate (2B) |
| Intranasal corticosteroidsa | Yes/Strong/High (1A) | No/Weak/Low (2C) |
| Intranasal formulation of anti-H1+corticosteroid (MP-AzeFlu)b | Yes/Weak/Moderate (2B) | No analysis |
| Oral corticosteroidsa | Yes/Weak/Very Low (2D) | No analysis |
| Subcutaneous immunotherapya | Yes/Weak/Moderate (2B) | Yes/Weak/Moderate (2B) |
| Sublingual immunotherapya (drops or tablets) | Yes/Weak/Moderate (2B) | Yes/Weak/Low (2C) |
| Omalizumab (anti-IgE)a | Yes/Weak/Moderate (2B) | Yes/Weak/Moderate (2B) |
| Intranasal decongestant ≤5 daysa (for nasal obstruction) | Yes/Weak/Very low (2D) | No analysis |
| Oral decongestanta (for nasal obstruction) | No/Weak/Low (2C) | No analysis |
| Intranasal ipratropiuma (for rhinitis) | Yes/Weak/Moderate (2B) | No analysis |
| Intranasal chromonea | Yes/Weak/Moderate (2B) | No analysis |
MP-AzeFlu: intranasal formulation of azelastine+fluticasone propionate
GRADE: Recommendation (Yes, No); Strength of recommendation (1, strong; 2, conditional); Level of scientific evidence (A, high; B, moderate; C, low; D, very low).
Scientific Evidence and Recommendations in the Comparison of Drugs for the Treatment of Allergic Rhinitis According to GRADE.
| Treatments Compared | Seasonal Allergic Rhinitis | Perennial Allergic Rhinitis |
|---|---|---|
| Combined oral anti-H1+intranasal corticosteroid vs intranasal corticosteroid alonea | Both [Yes/Weak/Low (2C)] | Intranasal corticosteroids only [Yes/Weak/Very Low (2D)] |
| Anti-H1+corticosteroid intranasal formulation (MP-AzeFlu) vs intranasal corticosteroid alonea | Both [Yes/Weak/Moderate (2B)] | Both [Yes/Weak/Very low (2D)] |
| Anti-H1+corticosteroid intranasal formulation (MP-AzeFlu) vs intranasal corticosteroid alonea | Intranasal formulation of anti-H1+corticosteroid [Yes/Weak/Low (2C)] | No analysis |
| Antileukotriene vs oral anti-H1a | Both [Yes/Weak/Moderate (2B)] | Oral anti-H1 [Yes/Weak/Low (2C)] |
| Intranasal anti-H1 vs intranasal corticosteroidsa | Intranasal corticosteroids [Yes/Weak/Moderate (2B)] | Intranasal corticosteroids [Yes/Weak/Low (2C)] |
| Oral anti-H1 vs intranasal anti-H1a | Both [Yes/Weak/Low (2C)] | Both [Yes/Weak/Very low (2D)] |
| Oral anti-H1(2nd generation) vs oral anti-H1 (1st generation)b | Oral anti-H1 (2nd generation) [Yes/Strong/Low (1C)] | Oral anti-H1 (2nd generation) [Yes/Strong/Low (1C)] |
| Intranasal corticosteroids vs oral anti-H1b | Intranasal corticosteroids [Yes/Weak/Low (2C)] | Intranasal corticosteroids [Yes/Weak/Moderate (2B)] |
| Intranasal corticosteroids vs anti-leukotrienesb | Intranasal corticosteroids [Yes/Strong/Low (1C)] | No analysis |
| Combined oral anti-H1+oral decongestant vs oral anti-H1b (continuous use) | Oral anti-H1 [Yes/Weak/Moderate (2B)] | Oral anti-H1 [Yes/Weak/Moderate (2B)] |
MP-AzeFlu: intranasal formulation of azelastine+fluticasone propionate.
GRADE: Recommendation (Yes, No); Strength of recommendation (1, strong; 2, conditional); Level of scientific evidence (A, high; B, moderate; C, low; D, very low).
Second-generation H1 antihistamines (bilastine, cetirizine, desloratadine, ebastine, fexofenadine, levocetirizine, loratadine, mequitazine, mizolastine, rupatadine) should preferably be used, rather than the first-generation products (sedatives), for improved effectiveness and, in particular, safety [strong recommendation; low-quality evidence] (Tables 1 and 2).35
Intranasal and Ocular Topical AntihistaminesTopical antihistamines (azelastine, emedastine, epinastine, levocabastine, olopatadine) have also been shown to be effective in rhinitis and allergic conjunctivitis [weak recommendation; low-quality evidence] (Table 1).35 They have a rapid onset of action (less than 15min).36 Oral antihistamines have shown greater effectiveness than topical products in the treatment of AR [weak recommendation; low-quality evidence] (Table 2).37
They are less effective than intranasal corticosteroids for the treatment of AR [weak recommendation; moderate-quality evidence] (Table 2).37
CorticosteroidsIntranasal CorticosteroidsIntranasal corticosteroids (INCS) (budesonide, ciclesonide, fluticasone propionate, fluticasone furoate, mometasone, triamcinolone) are very powerful anti-inflammatory drugs that are effective in the treatment of AR and also of NAR (fluticasone propionate), in both adults and children [strong recommendation; high-quality evidence] (Table 1),35 and are the most effective medications for the control of AR symptoms. In most studies, INCS have shown greater effectiveness than the combined use of an oral antihistamine with a leukotriene receptor antagonist in the treatment of AR [weak recommendation; low-quality evidence] (Table 2).35
The combination of a corticosteroid and an antihistamine (fluticasone propionate and azelastine) in an intranasal formulation (MP-AzeFlu) has demonstrated superior efficacy to the administration of each drug separately, with a very fast onset of action (5min). This formulation is currently indicated as first-line treatment for moderate-severe AR [weak recommendation; moderate-quality evidence] (Table 2).37,38
Oral CorticosteroidsThe use of short courses (1–3 weeks) of oral corticosteroids (prednisone, methylprednisolone, deflazacort) may be appropriate for the treatment of severe rhinitis that does not respond to other treatments [weak recommendation; very low-quality evidence] (Table 1).35
Nasal Decongestants (Oral and Intranasal)Intranasal decongestants (phenylephrine, nafazoline, oxymetazoline, tramazoline, xylometazoline) can be used for short periods (≤7 days) in patients with nasal obstruction. Their use in children is not recommended [weak recommendation; very low-quality evidence] (Table 1).35
Saline Nasal IrrigationThere is scientific evidence (systematic reviews and meta-analysis) that intranasal irrigation with saline is beneficial, and it is recommended in rhinitis and in CRS when used as single therapy or in combination with other treatments.
There are also a number of therapies that are common to both AR and allergic asthma: allergen avoidance, leukotriene receptor antagonists (montelukast), monoclonal antibodies (anti-IgE or omalizumab and other biologics), and subcutaneous or sublingual specific immunotherapy (drops or tablets).
Allergen AvoidanceAllergen avoidance is an accepted strategy, although it is controversial in the treatment of respiratory allergic diseases.39,40 In the case of allergy to pet dander, cockroaches, fungi and occupational agents, the effect of avoidance seems to be more obvious, although in many cases this strategy is difficult to implement [weak recommendation; very low-quality evidence].35
Anti-leukotrienesAnti-leukotrienes are less effective in monotherapy than oral antihistamines [weak recommendation; moderate-low-quality evidence] or intranasal corticosteroids [strong recommendation; low-quality evidence] in both adults and children (Table 2).35 In combination, they can enhance treatment with antihistamines and intranasal corticosteroids. They are a good alternative first-line therapy in patients with coexisting allergic rhinitis and asthma [weak recommendation; moderate-quality evidence] (Table 1).35,41
Specific ImmunotherapyThe indications for the use of specific immunotherapy (SIT) in asthma include suboptimal asthma control with medication or allergen avoidance, adverse drug effects, the patient's desire to avoid taking drugs, and the presence of comorbidities associated with asthma, especially allergic rhinitis. In allergic asthma, SIT has been shown to be effective in reducing the need for inhaled corticosteroids42 and in reducing rhinitis symptoms and the amount of medication needed to treat rhinitis [weak recommendation; moderate-quality evidence] (Table 1).35,43 Sublingual SLIT (drops or tablets) in patients with uncontrolled allergic asthma due to dust mites has recently been shown to reduce the number of exacerbations experienced during the inhaled corticosteroid reduction period.43 SIT has also been shown to be effective in patients with allergic rhinitis with sensitization to house dust mites, various pollens (olive, ragweed, grasses, Parietaria, cypress) and animal dander (dog and cat) [weak recommendation; moderate-quality evidence] (Table 1).35 It has the added advantage that the improvement persists for years, even after the cessation of immunotherapy.44
BiologicsOmalizumab or anti-IgE monoclonal antibody is indicated in the treatment of severe uncontrolled allergic asthma in patients over the age of 12 years [weak recommendation; moderate-quality evidence] (Table 1).35 Although omalizumab has also demonstrated its efficacy in allergic rhinitis [weak recommendation; moderate-quality evidence] (Table 1),35,45 it is not indicated in this disease because of its high cost.
Treatment of Non-allergic Rhinitis in Patients With AsthmaNAR presents with nasal symptoms similar to those of RA, but both skin and blood allergy tests are negative. It is not as well defined and its etiopathogenesis is diverse, with inflammatory, hormonal or neurogenic causes. For the same reason, the treatment of this presentation is not as well studied or standardized. Although its symptomatic treatment is similar to that described above for AR (antihistamines, nasal washes and intranasal or oral corticosteroids), the recommendations in NAR have not been assessed in clinical trials, and other treatments may vary if the etiology is known. In addition, we must bear in mind that NAR may mask CRS with or without nasal polyps, because the symptoms are indistinguishable.
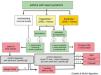
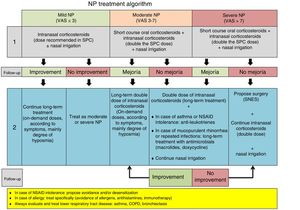
Treatment of Chronic Rhinosinusitis With Nasal Polyps in Patients With Asthma (Fig. 5)CRSwNP is a differentiated subtype of CRS with particular characteristics of severity and a tendency to recur (Fig. 5).
Treatment algorithm for chronic rhinosinusitis with nasal polyps. Outline of diagnosis, treatment, and monitoring of chronic rhinosinusitis with nasal polyps. COPD: chronic obstructive pulmonary disease; NSAIDs: non-steroidal anti-inflammatory drugs; NP: nasal polyps; NSES: sinonasal endoscopic surgery; VAS: visual analog scale of severity (0–10cm).
Intranasal corticosteroids are the only option, with a proven balance between effectiveness and optimal safety in the treatment of CRSwNP46 [Meta-analysis evidence].2 They improve the symptoms and quality of life of patients with concomitant asthma, but there is no evidence that they improve lung function.
Oral CorticosteroidsThe administration of oral corticosteroids (1–3 weeks) has been shown to be useful as rescue therapy in the treatment of CRSwNP (medical polypectomy) [Meta-analysis evidence].2 Long-term use is limited by the adverse effects, and these drugs must always be accompanied by prolonged treatment with intranasal corticosteroids.46
Nasal Washes With Saline SolutionSymptoms and quality of life of patients with NP improve with the administration of large volumes of hypertonic solutions (150ml or more), but no data show improvements in asthma. This treatment is practically free of side effects47 [Meta-analysis evidence].2
Second-line TreatmentSinonasal Endoscopic SurgerySinonasal endoscopic surgery (SNES) can be very conservative (polypectomy or simple excision of nasal polyps) or very radical (opening and cleaning of all the sinuses). It is indicated in patients who do not respond to medical treatment.
Although the level of scientific evidence is not high (IV), numerous series demonstrate the efficacy of surgery in CRSwNP, but its superiority to medical treatment has not been proven.2,48,49
Anti-leukotrienes (Montelukast)Adding montelukast to the core treatment of patients with CRSwNP has shown moderate effectiveness in improving symptoms, quality of life, and signs observed on nasal endoscopy and imaging studies (sinonasal CT), and in reducing the consumption of anti-asthma medications in patients with CRSwNP and asthma, but no improvement in lung function has been reported.2,50 Montelukast after surgery has not been shown to be superior to intranasal corticosteroids.
AntibioticsTreatment with oral antibiotics (erythromycin for 3 months or doxycycline for 21 days) has shown a slight but significant efficacy in the treatment of nasal polyposis (CRSwNP), and only erythromycin is effective in patients with asthma and CRSwNP.2,51
Biologics (Monoclonal Antibodies)A clinical trial with few patients and a case series with a low number of participants have demonstrated the positive effect of omalizumab (anti-IgE antibody) on symptoms, endoscopic signs, imaging studies (sinonasal CT), and quality of life in patients with CRSwNP and asthma, but no effect on lung function was reported.2,52
Both omalizumab and dupilumab (anti-IL-4Rα that blocks IL-4 and IL-13 pathways) and mepolizumab (anti-IL-5 antibody) have demonstrated efficacy in CRSwNP patients with and without asthma in phase 2 clinical trials. Omalizumab, dupilumab, mepolizumab, and benralizumab (anti-IL-5Rα) are currently being studied in CRSwNP patients with and without asthma in phase 3 clinical trials.
Aspirin DesensitizationDesensitization to aspirin by the oral route or by the intranasal application of lysine acetylsalicylate is an option in patients with NERD. It can only be performed in centers with experience in desensitization of this type, and the scientific evidence still lacks sufficient quality.2,53
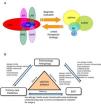
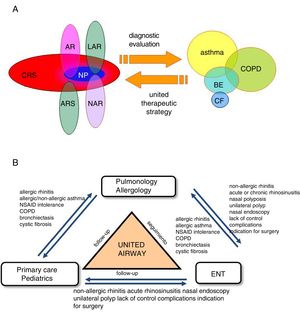
Comprehensive Management of Patients and Referral CriteriaMultidisciplinary management and referral criteria in patients with asthma and upper airway involvement (Fig. 6):
- •
The “united airways” approach usually requires multidisciplinary care, especially by ENT, allergology and pulmonology specialists, but also by pediatricians and primary care physicians.
- •
The criteria for consultation among specialists (ENT, allergology, respiratory medicine) depend on the ultimate goal of inter-department consultation and the availability of appropriate techniques for diagnosis and follow-up (Fig. 6B).
Comorbidities and multidisciplinary management of the united airways. (A) Sinonasal and bronchopulmonary comorbidities in the united airways concept. In addition to asthma, chronic obstructive pulmonary disease (COPD), bronchiectasis (BE), and cystic fibrosis (CF) can be associated with different diseases of the upper airway, such as allergic rhinitis (AR), local allergic rhinitis (LAR), non-allergic rhinitis (NAR), acute rhinosinusitis (ARS) or chronic rhinosinusitis (CRS) with or without nasal polyps (NP). A complete diagnostic evaluation and a unified therapeutic strategy are necessary for comprehensive management. (B) Multidisciplinary management of united airways. The airway is a common area that encompasses various medical specialties. Ideally, the different specialists involved (pulmonologists, allergologists, ENT specialists, primary care physicians or family doctors, and pediatricians) should collaborate in the diagnosis, treatment and follow-up of problems, in order to manage these patients comprehensively.
- 1.
All asthmatic patients must be studied for the presence of allergic or non-allergic rhinitis, and/or chronic rhinosinusitis with or without nasal polyps (1A), particularly in uncontrolled asthma.
- 2.
Diagnosis of allergic rhinitis in an asthma patient must be based on nasal symptoms and positive allergic skin prick tests and/or specific IgE antibodies to clinically relevant allergens in the blood (1A).
- 3.
Diagnosis of chronic rhinosinusitis with/without nasal polyps in an asthma patient must be based on nasal symptoms (including loss of smell), nasal endoscopy and/or sinonasal CT scan (1A).
- 4.
Mild allergic rhinitis in an asthma patient should be treated with antihistamines (1C), anti-leukotrienes (2A) or intranasal corticosteroids (1A).
- 5.
Moderate-severe allergic rhinitis in an asthma patient should be treated with intranasal corticosteroids (1A) or an intranasal formulation of corticosteroid+antihistamine (MP-AzeFlu) (2B).
- 6.
Specific immunotherapy may be recommended for allergic rhinitis in a patient with asthma who does not respond to medical treatment or in selected patients with a clinically significant allergen and corresponding symptoms (2B).
- 7.
Mild-moderate chronic rhinosinusitis with nasal polyps in patients with asthma should be treated with intranasal corticosteroids, in spray or drops, at full doses (2–4 times the dose for allergic rhinitis) (1A).
- 8.
In severe or exacerbating chronic rhinosinusitis with nasal polyps, the administration of no more than 3 courses of oral corticosteroids per year is recommended (1A), and the intranasal administration of corticosteroids must always be maintained.
- 9.
Sinonasal endoscopic surgery is recommended in asthma patients with chronic rhinosinusitis with severe nasal polyps or disease that cannot be controlled with medical treatment with intranasal and oral corticosteroids (1A). This indication may change in the near future due to the availability of biological drugs (1B).
- 10.
In our setting, we recommend desensitization to aspirin in asthmatic patients with NSAID-exacerbated respiratory disease (NERD) who require chronic anti-inflammatory treatment or antiplatelet therapy (e.g., cardioprotection). This should only be performed in expert centers (1B).
Dr. José Antonio Castillo Vizuete has collaborated with MSD, AstraZeneca, Boehringer Ingelheim, Uriach, GSK, Leti, and ALK.
Dr. Joaquim Mullol i Miret has been a member of national and international scientific advisory boards, and has received honoraria for lectures, grants for research projects funded by UCB Farma, Grupo Uriach SA, GSK, ALK-Abelló, Johnson & Johnson, and has acted as national coordinator or principal investigator in clinical trials for Allakos, ALK-Abelló, Mylan/MEDA Pharma, FAES, GSK, MSD, Sanofi-Genzyme & Regeneron, Genentech-Roche-Novartis, Hyphens, Pierre-Fabre, Menarini, and has received grants for research projects from Grupo Uriach SA and UCB Pharma.
Dr. Joaquín Sastre Dominguez has collaborated with Stallergenes, ALK-Abelló, Novartis, Sanofi, Roche, Faes Farma, GSK, Teva, and Mundipharma.
Dr. Alfonso del Cuvillo Bernal has no conflicts of interest.
Dr. César Picado Vallés has no conflicts of interest.
Dr. Eva Martínez Moragón has received speaker's honoraria from AstraZeneca, Chiesi, Teva and Novartis and consultant fees from ALK-Abelló, AstraZeneca and Boehringer Ingelheim.
Dr. Carolina Cisneros has received funding from AstraZeneca, Chiesi, Novartis, Teva, Mundifarma, GSK, Orionpharma, and Boheringer for lectures, research studies, and attendance at conferences and training courses.
Dr. José María Ignacio has received funding from AstraZeneca, Novartis, and GSK, for lectures, research studies, and attendance at conferences and training courses.
Dr. Francisco J. Álvarez Gutiérrez has no conflicts of interest.
Please cite this article as: Castillo Vizuete JA, Sastre J, del Cuvillo Bernal A, Picado C, Martínez Moragón E, Ignacio García JM, et al. Rinitis, poliposis nasal y su relación con el asma. Arch Bronconeumol. 2019;55:146–155.