The expansion of new tobacco consumption formats and electronic vapor products threatens to reverse the trend of declining smoking rates that had been observed among younger people in recent decades. Early detection in the health sector requires screening tools that have been adapted and validated in our context. This study aims to translate, culturally adapt, pilot and empirically validate the Hooked on Nicotine Checklist (HONC) with Spanish adolescents.
MethodsThe process of translation and cultural adaptation included the following stages: direct translation, synthesis of translations, back translation, consolidation with a committee of experts and pre-test, along with a cognitive interview, which served as pilot testing. Empirical validation was conducted with a sample of 1027 adolescents aged between 12 and 18 (M=15.40; SD=1.638).
ResultsThe results obtained confirm that the HONC is a brief, clear and easy-to-understand tool, with appropriate psychometric properties. A Cronbach's alpha value of 0.90 was obtained. Sensitivity, specificity, PPV and NPV indices reached values of 0.56, 0.94, 0.79 and 0.83, respectively. Cut-off point 1 is the one reaching the best balance between the two values. CFA showed a model good overall fit.
ConclusionThis study makes the Spanish version of the HONC available to researchers and clinicians, so that it can be used with sufficient psychometric guarantees.
Around 7 million smokers die each year as a direct consequence of tobacco consumption,1 and it is estimated that this figure will exceed 8 million by the year 2030.2 In Spain, tobacco remains the leading cause of preventable death.3
Adolescence is a critical period for the onset of smoking. According to the latest edition of the HBSC (Health Behavior in School-aged Children) study, 28% of 15-year-old adolescents in Spain have already tried tobacco at least once.4 According to official data, in 2021, 169,600 young people in Spain started smoking (74,500 boys and 95,100 girls).5 The average age at which Spaniards start smoking has been around 14 years since 2014, and the habit of daily consumption is acquired around 14.6 years.6 Initiating smoking at such an early age involves greater exposure to harmful components and increases susceptibility to the harmful effects of tobacco smoke, extending the potential duration of smoking throughout life and increasing the risk of chronic diseases related to its consumption.7 Although conventional cigarettes continue to be the predominant form of consumption, in recent years, other formats such as hookahs8,9 or electronic cigarettes or vaping devices10 have gained prominence. According to the latest Youth Risk Behavior Survey report, the use of vapor electronic products is already considered a public health problem in the United States, as 36.2% of high school students claim to have vaped nicotine at some point in their lives, and 18% have done so in the last 30 days.11 In Spain, the situation seems even more alarming, as the 2023 ESTUDES survey report indicates that 54.3% of students aged 14 to 18 have tried electronic cigarettes at least once in their lives, and 26.3% have done so in the last month. This represents an increase of 10 and 20 percentage points, respectively, compared to 2021 data.6 Experts agree that the impact of these new modalities threatens to reverse the trend of reduced tobacco consumption observed among young people in recent decades.12 For all these reasons, the World Health Organization (WHO), through the Framework Convention on Tobacco Control, emphasizes the concern about tobacco and nicotine consumption in children and adolescents, highlighting the need for intervention at an early age.13 As a result, some authors have already emphasized the need for a paradigm shift in addressing the problem, advocating for early detection and intervention in consumption from a healthcare perspective, under the umbrella of the well-known SBIRT (Screening, Brief Intervention, and Referral to Treatment) model.14 In this context, one of the most widely used screening tools globally is the CRAFFT Substance Abuse Screening Test developed by Knight et al. in 199915 and validated with Spanish adolescents by Rial et al. in 2019.16 However, the changing landscape posed by the emergence of new tobacco/nicotine consumption modalities justifies researchers from the Center for Adolescent Behavioral Health Research (CABHRe) (Boston, USA) redirecting their efforts toward adapting and updating this screening tool. This has resulted in the CRAFFT 2.1+N,17 a new version of the instrument that includes a specific screening item for tobacco or vaping use and incorporates, as an additional section, the Hooked on Nicotine Checklist (HONC).18 The HONC was developed in 2002 by researchers from the University of Massachusetts, in collaboration with researchers from Harvard University and St. George's Hospital in London, to determine the onset and intensity of tobacco dependence.18 It is a brief and simple questionnaire (consisting of 10 dichotomous items) that can be self-administered or completed during an interview. Despite its potential for early detection of tobacco/nicotine dependence among young people and adolescents,19 there is very little empirical research supporting its psychometric properties in the Spanish context. Therefore, the present study aims at adapting and validating the HONC with Spanish adolescents. The goal is to translate, culturally adapt, pilot, and empirically validate the scale so that it can be used with confidence.
MethodsProcedureIn PHASE 1, the process of translation and cultural adaptation was carried out, including the adaptation of items, questionnaire instructions, and response options.20 To ensure semantic, idiomatic, conceptual, and experiential equivalence of the instrument concerning the original test, specific methodological recommendations for health questionnaires21 and guidelines for the transcultural adaptation process of self-report measures20 were followed. The process included direct translation (by bilingual researchers), synthesis of translations, back-translation (by professional bilingual translators), consolidation with an expert committee (comprising an expert in test methodology and validation, six addiction experts from different regions of Spain, and members of the research team), and pre-testing. This last stage aimed at piloting the prefinal version with a small sample of the target population,20 thus evaluating the quality of translation, cultural adaptation, questionnaire feasibility, and required completion time.21 This pilot was conducted in both interview and self-administered questionnaire formats. Finally, a report summarizing the results was prepared and served as the basis for final decisions.
In PHASE 2, the empirical validation of the tool was carried out. The HONC was included among a battery of self-administered screening questionnaires used in a data collection conducted in educational centers as part of a larger study, which also included the validation of this tool among its objectives.
The study obtained consent and collaboration from both the school authorities and the parents of the participating minors. Participation was voluntary and unpaid, and participants were informed of the purpose of the data collection, ensuring the confidentiality and anonymity of responses. The study was approved by the Bioethical Committee of the University of Santiago de Compostela.
InstrumentsFor Phase 1 (pilot test), a custom questionnaire was designed, consisting of three blocks. The first block included the Spanish translation of the HONC. In the second block, as a cognitive interview (using the probing-based paradigm – retrospective probing procedure), four questions were included to assess the degree of understanding of the tool, the clarity of its items, and any potential questions or difficulties in understanding that the instrument might raise. Finally, a sociodemographic block was included (gender, age, and grade). All questionnaires included a section to record the duration of each interview or the completion time of each self-administered questionnaire.
As for Phase 2 (empirical validation), data were collected through a custom-designed questionnaire. In this case, in addition to a sociodemographic section (gender, age, grade, and educational center) and the translated version of the HONC, the Spanish version of the Substance Use and Abuse subscale of the Problem Oriented Screening Instrument for Teenagers (POSIT-UAS)22 was included, which was to be used as the Gold Standard (cut-off point 2).
ParticipantsFor participant selection in Phase 1, accidental sampling was used. A total of 46 adolescents completed the self-administered questionnaire, while 35 were interviewed.
In Phase 2, a selective methodology was employed, involving the administration of questionnaires to students in Compulsory Secondary Education (ESO), Baccalaureate (BAC), and Vocational Training (FP) in the autonomous community of Galicia (Spain). A purposive sampling method was used to select the sample, with a total of 11 educational centers collaborating. Participants had to be students aged between 12 and 18 years. Exclusion criteria included refusal to participate and the presence of a high percentage of missing values or an inconsistent response pattern. The initial sample consisted of 1089 adolescents, although 62 were excluded for not meeting the inclusion criteria or presenting any exclusion criteria. To ensure that there was no bias in the distribution of missing cases and that these were randomly distributed, it was confirmed that the percentage of missing cases was similar in different sample segments based on gender, age group, and school ownership. For this purpose, χ2 contrast statistics were calculated.
Data AnalysisFor the pilot test, a descriptive analysis of the data was conducted by calculating percentages. For empirical validation, first, a descriptive analysis was performed by calculating percentages and statistics for central tendency and dispersion. To assess internal consistency, Cronbach's Alpha coefficient (α) (KR-20) was calculated. The Corrected Homogeneity Index (CHI) for each item was also calculated, along with α values when the item was removed. To check dimensionality, a Confirmatory Factor Analysis (CFA) was carried out using the Unweighted Least Squares (ULS) method for parameter estimation. Finally, to assess screening capacity, the POSIT-UAS was used as the Gold Standard, allowing the calculation of sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for different cut-off points.
ResultsTable 1 shows the HONC final version in Spanish, resulting from the translation and validation process.
Spanish HONC Final Version.
| HONC |
|---|
| Las siguientes preguntas tratan sobre tu consumo de tabaco y/o sobre tu uso de vapers con nicotina y/o sabores. |
| 1. ¿Alguna vez has intentado dejar de consumirlo, pero no fuiste capaz? |
| 2. ¿Te resulta tan difícil dejarlo que por ello consumes tabaco o vapeas actualmente? |
| 3. ¿Alguna vez has sentido que eras adicto/a al tabaco o al vapeo? |
| 4. ¿Alguna vez has sentido un deseo muy intenso e irrefrenable de consumir tabaco o vapear? |
| 5. ¿Alguna vez has sentido que realmente necesitabas consumir tabaco o vapear? |
| 6. ¿Se te hace duro no consumir tabaco o no vapear en lugares donde no debes hacerlo, como por ejemplo en el colegio/instituto? |
| 7. Cuando no has consumido tabaco o vapeado durante un rato (o cuando has intentado dejarlo)… |
| a. ¿te resultaba difícil concentrarte porque no podías consumir tabaco o vapear? |
| b. ¿te sentías más irritable porque no podías consumir tabaco o vapear? |
| c. ¿sentías una fuerte necesidad o deseo de consumir tabaco o vapear? |
| d. ¿te sentías nervioso/a, inquieto/a o ansioso/a porque no podías consumir tabaco o vapear? |
The participants’ age in the pilot test of the self-administered version of the HONC (n=46) ranged from 12 to 16 years old (M=13.36; SD=1.190). 47.8% were female, and 50% were in the first cycle of ESO, while the other 50% were in the second cycle. Participants in the interview version pilot test (n=35) also ranged from 12 to 16 years old (M=13.63; SD=1.629), of which 25.7% were female, with 57.1% in the first cycle of ESO and 42.9% in the second cycle.
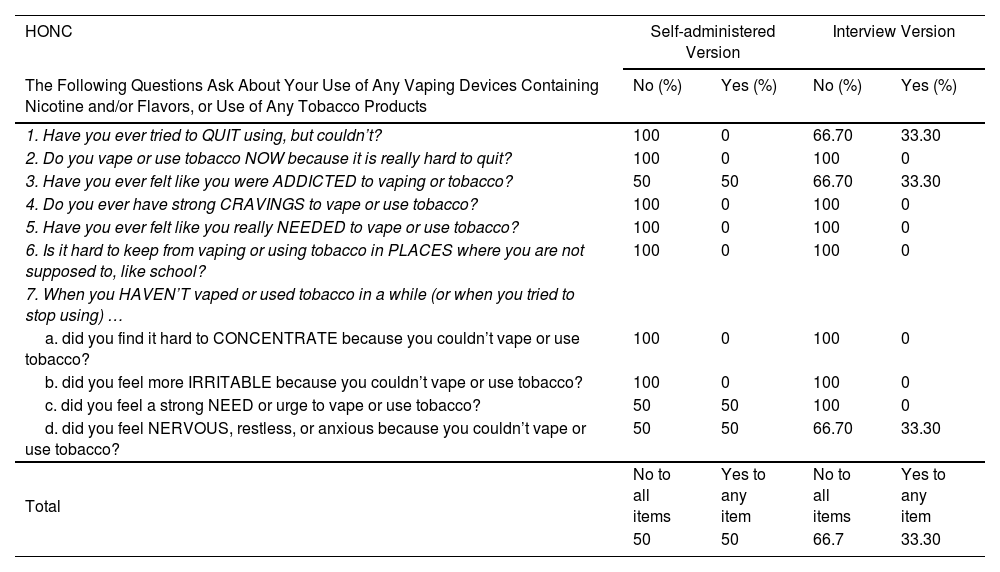
The completion time for both versions ranged from 2 to 5min, with an average of 2.46min for the self-administered version and 2.21min for the interview version. Table 2 shows the percentage of positive and negative responses for each HONC item in each administered version.
Percentage of Affirmative and Negative Responses for Each HONC Item Obtained in the Pilot Test (Self-administered Version and Interview).
| HONC | Self-administered Version | Interview Version | ||
|---|---|---|---|---|
| The Following Questions Ask About Your Use of Any Vaping Devices Containing Nicotine and/or Flavors, or Use of Any Tobacco Products | No (%) | Yes (%) | No (%) | Yes (%) |
| 1. Have you ever tried to QUIT using, but couldn’t? | 100 | 0 | 66.70 | 33.30 |
| 2. Do you vape or use tobacco NOW because it is really hard to quit? | 100 | 0 | 100 | 0 |
| 3. Have you ever felt like you were ADDICTED to vaping or tobacco? | 50 | 50 | 66.70 | 33.30 |
| 4. Do you ever have strong CRAVINGS to vape or use tobacco? | 100 | 0 | 100 | 0 |
| 5. Have you ever felt like you really NEEDED to vape or use tobacco? | 100 | 0 | 100 | 0 |
| 6. Is it hard to keep from vaping or using tobacco in PLACES where you are not supposed to, like school? | 100 | 0 | 100 | 0 |
| 7. When you HAVEN’T vaped or used tobacco in a while (or when you tried to stop using) … | ||||
| a. did you find it hard to CONCENTRATE because you couldn’t vape or use tobacco? | 100 | 0 | 100 | 0 |
| b. did you feel more IRRITABLE because you couldn’t vape or use tobacco? | 100 | 0 | 100 | 0 |
| c. did you feel a strong NEED or urge to vape or use tobacco? | 50 | 50 | 100 | 0 |
| d. did you feel NERVOUS, restless, or anxious because you couldn’t vape or use tobacco? | 50 | 50 | 66.70 | 33.30 |
| Total | No to all items | Yes to any item | No to all items | Yes to any item |
| 50 | 50 | 66.7 | 33.30 | |
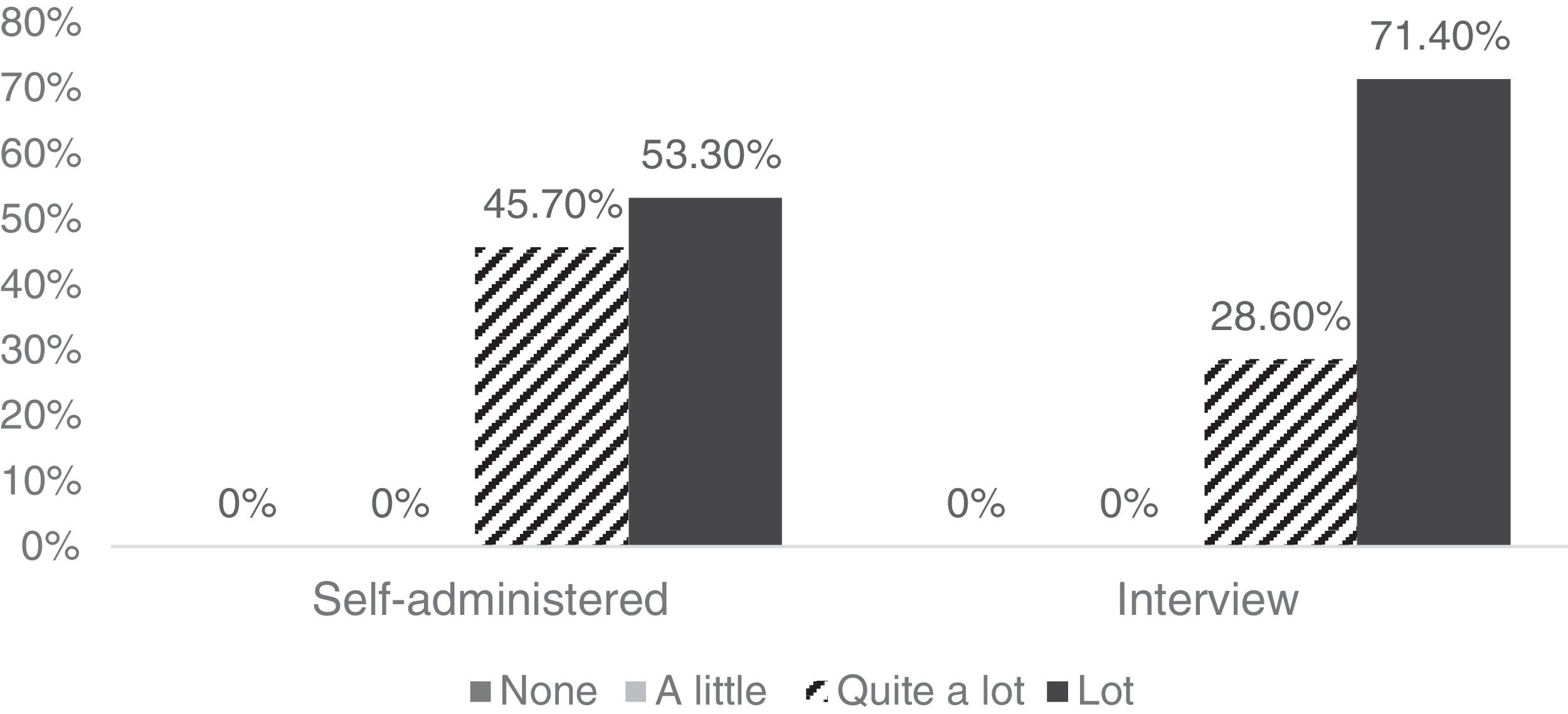
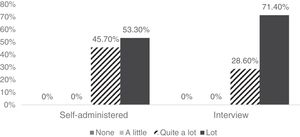
The 81 participants considered the tool to be quite or very clear, both in the self-administered version and the interview format (Fig. 1). Also, 97.8% reported not having difficulties understanding the items in the self-administered version, and 97.1% reported the same for the interview version.
In Phase 2, 1027 students aged 12–18 years (M=15.40; SD=1.638) were included. 54.6% were studying ESO, 37.2% BAC, and 8.2% FP. Females accounted for 44.7%. Out of all participants, 22.88% (235 subjects) reported having smoked or used some nicotine and/or flavored vaping device in the last 12 months and 15.91% in the last month, placing the age onset tobacco use at 14.23 years. More specifically, 36.12% of those who reported having used tobacco/nicotine in the last month used only conventional cigarettes, 24.43% only vapes or electronic cigarettes and the remaining 39.45% were dual smokers. Table 3 shows the direct responses to each HONC item.
Percentage of Affirmative and Negative Responses to Each HONC Item Obtained in the Empirical Validation Process (n=235).
| HONC | Self-administered version | |
|---|---|---|
| The Following Questions Ask About Your Use of Any Vaping Devices Containing Nicotine and/or Flavors, or Use of Any Tobacco Products | No (%) | Yes (%) |
| 1. Have you ever tried to QUIT using, but couldn’t? | 75.30 | 24.70 |
| 2. Do you vape or use tobacco NOW because it is really hard to quit? | 78.70 | 21.30 |
| 3. Have you ever felt like you were ADDICTED to vaping or tobacco? | 71.50 | 28.50 |
| 4. Do you ever have strong CRAVINGS to vape or use tobacco? | 57.90 | 42.10 |
| 5. Have you ever felt like you really NEEDED to vape or use tobacco? | 58.30 | 41.70 |
| 6. Is it hard to keep from vaping or using tobacco in PLACES where you are not supposed to, like school? | 81.30 | 18.70 |
| 7. When you HAVEN’T vaped or used tobacco in a while (or when you tried to stop using) … | ||
| a. did you find it hard to CONCENTRATE because you couldn’t vape or use tobacco? | 86.80 | 13.20 |
| b. did you feel more IRRITABLE because you couldn’t vape or use tobacco? | 79.10 | 20.90 |
| c. did you feel a strong NEED or urge to vape or use tobacco? | 71.90 | 28.10 |
| d. did you feel NERVOUS, restless, or anxious because you couldn’t vape or use tobacco? | 76.20 | 23.80 |
| Total | No to all items | Yes to any item |
| 5.50 | 95.50 | |
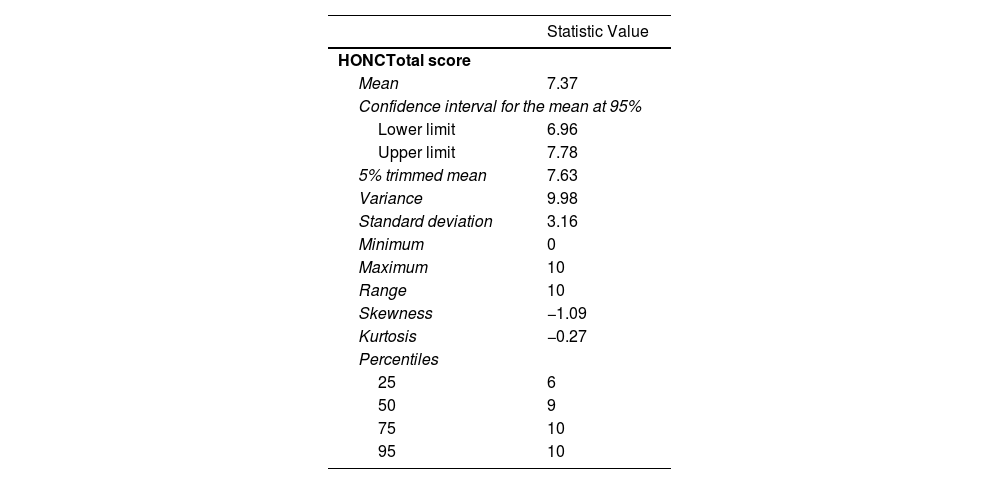
Descriptive statistics for the total HONC score are presented in Table 4. Standardized skewness and kurtosis statistics reveal negative skewness and a platykurtic distribution, indicating that the scores do not follow a normal distribution. The violation of normality was tested using the Kolmogorov–Smirnov test, with Lilliefors correction (K–S=0.220; p<0.001).
Descriptive Data for the HONC Total Score.
| Statistic Value | |
|---|---|
| HONCTotal score | |
| Mean | 7.37 |
| Confidence interval for the mean at 95% | |
| Lower limit | 6.96 |
| Upper limit | 7.78 |
| 5% trimmed mean | 7.63 |
| Variance | 9.98 |
| Standard deviation | 3.16 |
| Minimum | 0 |
| Maximum | 10 |
| Range | 10 |
| Skewness | −1.09 |
| Kurtosis | −0.27 |
| Percentiles | |
| 25 | 6 |
| 50 | 9 |
| 75 | 10 |
| 95 | 10 |
Using the original scale cut-off point (≥1), it should be noted that 21% of the total study participants (n=1027) tested positive, equivalent to 94.5% of the subsample of smokers (n=235). When comparing mean scores by gender, females had a lower score than males (7.05 vs. 7.85), but this difference was not statistically significant (t=−1.913; p=0.057; Z=−1.86; p=0.062). Regarding age, differences among the three established groups (12–14, 15–16, and 17–18 years old) were not statistically significant (F=0.633; p=0.532; H=1.267; p=0.531). Given the non-normality in the distribution of HONC scores, non-parametric tests (Mann–Whitney and Kruskal–Wallis) were also used, yielding similar results.
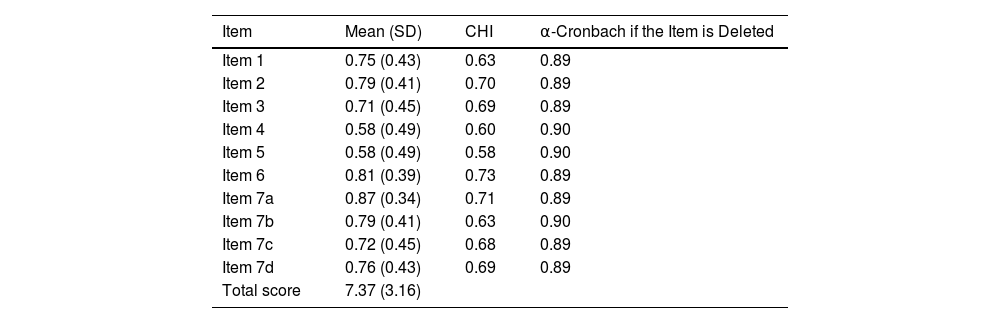
To analyze internal consistency, Cronbach's Alpha coefficient (KR-20) was calculated, resulting in a value of 0.90. The Corrected Homogeneity Index for each item (CHI) was also calculated, with values ranging from 0.581 to 0.727. Items 4 and 5 showed the least consistency with the overall scale. However, removing either of them did not improve overall consistency (Table 5).
Consistency of HONC Items.
| Item | Mean (SD) | CHI | α-Cronbach if the Item is Deleted |
|---|---|---|---|
| Item 1 | 0.75 (0.43) | 0.63 | 0.89 |
| Item 2 | 0.79 (0.41) | 0.70 | 0.89 |
| Item 3 | 0.71 (0.45) | 0.69 | 0.89 |
| Item 4 | 0.58 (0.49) | 0.60 | 0.90 |
| Item 5 | 0.58 (0.49) | 0.58 | 0.90 |
| Item 6 | 0.81 (0.39) | 0.73 | 0.89 |
| Item 7a | 0.87 (0.34) | 0.71 | 0.89 |
| Item 7b | 0.79 (0.41) | 0.63 | 0.90 |
| Item 7c | 0.72 (0.45) | 0.68 | 0.89 |
| Item 7d | 0.76 (0.43) | 0.69 | 0.89 |
| Total score | 7.37 (3.16) |
To analyze the scale dimensionality, a CFA was conducted using the ULS method for parameter estimation. As a result, the unifactorial structure proposed by the original authors17 was confirmed. The model fit was good, both structurally (all items showed statistically significant and above 0.80 factor loadings) and globally, with fit indices above 0.90 (GFI=0.999; AGFI=0.999; NFI=0.999).
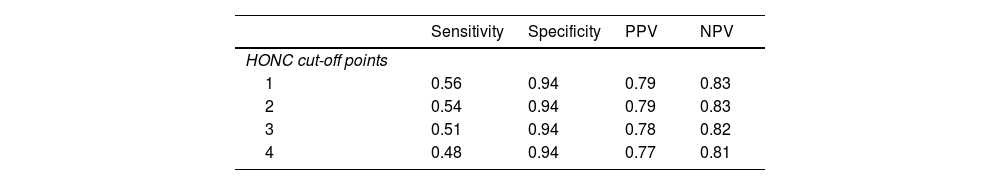
To analyze screening capacity, sensitivity, specificity, PPV, and NPV were calculated for different cut-off points, using POSIT-UAS as the Gold Standard. As shown in Table 6, sensitivity values are very low for the four analyzed cut-off points. However, results are very acceptable in terms of specificity, PPV, and NPV, with values even above 0.90 for specificity. Cut-off point 1 achieves the best balance among the four indicators.
Lastly, to provide evidence related to criterion validity, the correlation between overall HONC scores and POSIT-UAS was calculated. Pearson's R coefficient and Spearman's Rho were significant in both cases (p<0.01) (0.50 and 0.51, respectively). Additionally, the Kappa index was calculated to study the agreement in screening performed by both instruments, reaching a value of 0.53 (p<0.01).
DiscussionCurrent prevalence rates of tobacco and electronic vapor product use among adolescents necessitate health professionals to have brief screening tools that have demonstrated psychometric properties and can detect tobacco/nicotine dependence early. The HONC has been widely used for detecting tobacco/nicotine dependence among adolescents,23–25 however, it has not been validated yet in Spain, despite recommendations from the Spanish Association of Primary Care Pediatrics since 2009.26
The rigorous translation and cultural adaptation process in this study, along with the pilot test results, demonstrate that the HONC is a brief, clear, and easy-to-understand tool. In none of the questions, the percentage of participants encountering difficulties exceeded 15%, so no revisions were necessary.21 Thus, the final versions do not imply any changes from the piloted versions.
Throughout the empirical validation phase, its psychometric performance as a mass screening tool was studied. Consistent with the original authors’ findings,18 the results indicate that the optimal cut-off point for detecting nicotine dependence problems among Spanish adolescents aged 12–18 is ≥1. Also, from the perspective of internal consistency, it has been demonstrated that the HONC exhibits excellent reliability. The obtained coefficient alpha (0.90) is high, similar to27 or even higher28 than calculated in other studies.
Few authors have previously addressed the scale's dimensionality. In this regard, this study has updated the available evidence and confirmed the unidimensionality proposed by the original authors.18 Likewise, a good fit of the model has been demonstrated, both structurally and globally.
Finally, it is important to note some limitations. Despite having a sample of 1027 adolescents, the inability to use a probabilistic sampling strategy and ensure proportional allocation by variables such as “age” means that the estimated figures should be interpreted with caution. The fact that only adolescents from a single autonomous community were used constitutes a limitation to external validity and makes broader studies at the national level desirable. All collected variables have been self-reported, so the responses may depend on the subjectivity of the informant. However, different experts have pointed out that self-report measures are reliable and even preferable when assessing substance use habits in youth and adolescents.29,30 The obtained results indicate that the HONC is a tool with low sensitivity to detect tobacco/nicotine dependence. This may be due to using an inappropriate Gold Standard. The POSIT-UAS is a screening tool for general substance risk consumption that can yield positive results due to the use of substances other than tobacco (e.g., alcohol). This would also explain the correlation and agreement index values between the overall scores of HONC and POSIT-UAS, which barely exceed 0.50. In future studies, it would be advisable to use a Gold Standard that allows for a more precise determination of the screening capacity for tobacco/nicotine dependence.
In conclusion, this study provides clinicians and researchers with a Spanish-translated and culturally adapted version of the HONC. It offers evidence regarding its psychometric properties for screening tobacco/nicotine dependence. To use it confidently with Spanish adolescents, a cut-off point of ≥1 is recommended. Despite the mentioned limitations, the HONC proves to be a valid and reliable tool suitable for use in early detection programs and brief counseling.14
FundingThis study was supported by the Spanish National Plan on Drugs (File 2018/008).
Conflict of InterestThe authors declare no conflicts of interest.
The authors express gratitude to the group of experts who contributed to the consolidation phase of this study.