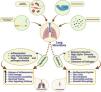
Lung cancer remains the leading cause of cancer-related deaths worldwide. According to the American Cancer Society (ACS), it ranks as the second most prevalent type of cancer globally. Recent findings have highlighted bidirectional gut–lung interactions, known as the gut–lung axis, in the pathophysiology of lung cancer. Probiotics are live microorganisms that boost host immunity when consumed adequately. The immunoregulatory mechanisms of probiotics are thought to operate through the generation of various metabolites that impact both the gut and distant organs (e.g., the lungs) through blood. Several randomized controlled trials have highlighted the pivotal role of probiotics in gut health especially for the prevention and treatment of malignancies, with a specific emphasis on lung cancer. Current research indicates that probiotic supplementation positively affects patients, leading to a suppression in cancer symptoms and a shortened disease course. While clinical trials validate the therapeutic benefits of probiotics, their precise mechanism of action remains unclear. This narrative review aims to provide a comprehensive overview of the present landscape of probiotics in the management of lung cancer.
Recent studies have suggested that lung cancer ranks as the second highest cause of mortality worldwide.1 Several authors have established that smoking accounts for over 90% of lung cancer incidences. In addition, air pollution and several other respiratory toxins are being recognized as important risk factors for lung carcinogenesis. There are several factors that largely influence the pathological condition of a disease, namely, the influx of bacterial colonies, immunological removal of such colonies, adaptability of the bacterial strains to host threat, and the capability of such microbes to reproduce under specific environmental conditions. Although the health of individuals with early-stage lung cancer has significantly improved during the past few decades, the total 5-year survival rate is reported to be just over 9% for males and 15% for women, despite developments and numerous treatment advancements.2 Hence, innovative and advanced approaches are needed to prevent and treat lung cancer.3 Available evidence has put forward that except for tobacco smoking, which is a well-known risk factor for lung cancer, several other risk factors can be involved, including genetic factors, infections (HPV, HIV, and EBV), previous lung disease (e.g., chronic obstructive pulmonary disease, tuberculosis), air pollution, radon or asbestos exposure, improper diet, marijuana smoking, and alcohol consumption.4 However, there are cases with lung cancer who have never smoked and do not have the aforementioned risk factors. This issue has sparked curiosity among researchers worldwide to explore other factors involved in the pathogenesis of lung cancer.
Trillions of microorganisms have been reported to reside within the human gastrointestinal system, and the bacterial interplay between the microbiota and other body cells is crucial for preserving immunological homeostasis in the gut milieu.5–7 The World Health Organization (WHO) and the Food and Agriculture Organization (FAO) have defined probiotics as live microorganisms that bestow a favorable impact on human health when administered adequately.8,9 Potential nutritional and physiological benefits of gut microbiota include nutrient utilization, infection resistance, modification of gut microbial groups, immune reinforcement, and regulation of the host metabolism.10 Probiotics that boost hosts’ health have garnered much attention recently. Clinical studies have revealed that probiotics can improve immune function and reduce the severity and incidence of lung diseases.11 The idea of regulating immunomodulation and governing pulmonary homeostasis through healthy bacteria by gut–lung interaction is reinforced using beneficial probiotics.
In conclusion, while lung cancer remains a formidable global health challenge with a low 5-year survival rate, the multifaceted nature of its development, involving various risk factors such as smoking, genetic predisposition, and environmental exposures, necessitates innovative approaches. Beyond traditional risk factors, the emerging field of research on the gut–lung interaction highlights the potential of probiotics in modulating immune function and influencing lung health, providing a promising avenue for further exploration and the development of novel strategies for lung cancer prevention and management.
This review aims to comprehensively examine the role of probiotics in the development, progression, and treatment of lung cancer, with a specific focus on their impact through the gut–lung axis and immune modulation. We seek to explore the potential mechanisms by which probiotics may influence lung cancer biology, acknowledging the current gaps in understanding their precise mechanisms. Additionally, this review will identify key areas for future research, particularly the need for clinical trials that delineate the efficacy of specific probiotic strains, optimal dosages, and treatment durations. Recognizing the variability in individual responses to probiotics, the prospects for personalized probiotic therapies tailored to individual genetic backgrounds, microbiome compositions, and lifestyle factors will also be considered. Furthermore, the integration of probiotics with conventional lung cancer treatments, evaluating potential synergistic effects, safety concerns, and regulatory considerations will also be discussed. By doing so, this review aims to shed light on the promising yet complex role of probiotics in lung cancer management, setting the stage for future investigations that could lead to novel therapeutic strategies.
MethodsThe relevant literature was searched using Web of Science, PubMed, and Science Direct in English by December 2023. The databases were searched using the following keywords: “probiotics”; “microbiome”, “lung cancer”, “gut–lung axis”, “immune system” and their equivalent terms. Case reports, commentary, and Editorial papers were not included in this review.
Gut–lung axisThe gastrointestinal tract and the lungs share a common embryonic origin.1 In addition, they can physically interact due to the possibility of ingested germs reaching the gastrointestinal and respiratory systems, as well as the ability of gastroesophageal contents to enter the lungs through inhalation.12 The gut–lung axis (GLA) has been identified as playing a critical role in both health and disease, underpinned by the extensive interaction between gastrointestinal and pulmonary systems.13 Similarities in the mucosa covering the stomach and lungs and those of the other organs that make up the mucosal immune system (MIS), foster comparable dynamics involving exchanges between the immune system and associated microbiota.14 As a result of their indirect connections with the lymphatic and circulatory systems, the immune response also influences the scenario systematically; however, the extent of immunological reaction is mostly dependent on the location of the very first encounter.15 Firmicutes and Bacteroidetes are the common bacterial phyla in the lungs and intestines of healthy humans. The overall microbial ecology is influenced by the interplay between lungs and intestines, partly because of nutrient interchange and antimicrobial bio-actives.16–18 The relationship between the microbial population of the lung and the gut is vague, and how the gut microbiota impacts the lungs, intestine, and other systems is a topic of further investigation.19 Antigen-presenting cells (e.g., dendritic cells), B cells, T cells (esp. Treg cells), and intestinal epithelial cells are among the most stimulated cells by microbial communities.20 There have been several progressive studies explaining the molecular foundation of entero-pulmonary interactions. These studies have mainly reported on antimicrobial peptides (AMPs) and secretory IgA, as well as the formation of microbial metabolites like the kynurenine pathway.21 The incidence of asthma, lung infections, and several other respiratory diseases have now been linked to dysbiosis.22 Brown et al. revealed the physiological benefits of the gut microbiota.23 Several gut microbiotas are involved in the synthesis of interleukins, like IL-17A, which is essential for the increased production of granulocyte-macrophage-stimulating factors in the lung. These factors activate the killing and elimination of potential macrophages in the lung alveoli.24 Furthermore, lung microbiota modification causes both local and systemic immune alterations. These events may promote intestinal immune response, as seen in Staphylococcus aureus-induced pneumonia, resulting in sepsis and apoptotic events in the gut.25
Lung microbiomes and host immunityThe mucus layer in the intestine acts as a physical barrier that separates the luminal contents of the gut, including digested food and microorganisms, from the underlying epithelial cells of the intestinal lining. This barrier helps prevent direct contact between potentially harmful bacteria and the epithelium, which can trigger inflammatory responses or infections. The mucus layer also contains immunoglobulins, antimicrobial peptides, and other immune components that help defend against invading pathogens. Additionally, the mucus layer serves as a habitat for certain beneficial bacteria, which can compete with harmful bacteria for resources and space.26 The recent identification of a novel CD4+ T cell subset known as Th17 has revolutionized the comprehension of the underlying causes of numerous chronic immune-mediated conditions. Particularly in regions of the body that interact closely with the microbial environment, such as the gastrointestinal and respiratory tracts, as well as the skin, where Th17 cells are predominantly found. When the immune response against self-components becomes dysregulated in these tissues, it can lead to the development of chronic inflammatory diseases.27 By influencing the host's sensitivity to different pathogenic agents and therapeutic effects, the microbiota controls the host's immune functionality. The ability of hosts to detect and stop bacterial or fungal invasions and illnesses results from the dynamic interplay between the microbiota and the immune systems. Secretory IgA (SIgA) plays a vital role as the initial defense barrier safeguarding the intestinal epithelium against enteric toxins and pathogenic microorganisms. Through a process referred to as immune exclusion, SIgA actively contributes to the removal of antigens and pathogenic microorganisms from the intestinal lumen. It achieves this by obstructing their access to epithelial receptors, ensnaring them within the mucus layer, and facilitating their elimination through peristaltic and mucociliary activities.28 In the human intestine, a stable state of equilibrium is upheld through precise regulation of microbial levels and the corresponding immune response. When this balance falters, it can lead to various pathological conditions. Toll-like receptors (TLRs), which are a part of the innate immune system, play a pivotal role as intermediaries connecting the intestinal epithelial barrier, the microbiota, and the immune system. The TLR pathway, which is typically activated in response to pathogens, contributes to the development of numerous infectious and inflammatory diseases.29 Preclinical studies on germ-free mice have shown significant immunological abnormalities, including a compromised mucous layer, underlining the gut microbiota's role in immune homeostasis. This effect may be attributed to reduced immunoglobulin secretion.30,31 IgA reportedly controls bacterial pathogenicity in the stomach primarily by preventing bacterial adhesion to mucosal epithelial cells.28 Interleukin 17 (IL-17) stands as a crucial proinflammatory cytokine within the T helper 17 (Th17) pathways. It plays a pivotal role in both the clearance of specific pathogens and the development of various inflammatory diseases. This cytokine, along with its counterpart IL-17F, is expressed by distinct types of T cells, particularly Th17 cells, as well as certain other lymphocytes. These cytokines are central players in regulating the immune response during host defense and in the context of various inflammatory disorders.32 The delicate balance of commensal bacteria is more likely to be upset and exogenous pathogens are more likely to infect low-density micro-ecologies with unstable microbiomes.33 High commensal microbiota density may improve the clinical effectiveness of vaccination in infants.30 The clearance of certain pathogens is aided by IL-17, where the gut microbiota may control and boost Th17 responses.34,35 IL-17 pathway is associated with various diseases such as airway disease (asthma and obliterative bronchiolitis), sarcoidosis, and bone marrow transplant-related pneumonitis.10,36–38 Iida et al. reported that microbiota of lungs regulates gene expression of various inflammatory cytokines (IL-5, IL-10, and IFN). Furthermore, their experiments on lungs of neonatal mice showed programmed death ligand 1 (PD-L1) which was expressed at a higher level as compared to germ-free mice. There was also increase in the number of CD11b+dendritic cells (DCs) and FoxP3+CD25+Treg cells.39 Steed et al. demonstrated that desaminotyrosine (DAT), a metabolite produced by Clostridium orbiscindens, defends the host from influenza by increasing the type I IFN signaling and thereby decreasing lung cancer pathogenesis.40 Ichinohe et al.41 also reported data showing that commensal microbiota might regulate immune responses in airway mucosa by inflammasomes and convey activation signals (immune signals) after influenza virus infection remains in a steady state. Inulin, a fermentable fiber, has been reported to change the composition of the gut microbiome and related metabolites, including short chain fatty acids. It is also found to promote resistance to influenza-virus infection via decreasing neutrophil-induced injury and promoting the antiviral CD8+ T cell responses in mice. Additionally, enriched lung microbiota due to oral microbiomes can be related to Th17 inflammation. The composition of the lung microbiota is shown to critically affect TLR4 responses.25 Additionally, it has been demonstrated that the commensal microbiota promotes the growth and activation of Vg6+Vd1+ T cells in lung cancer.41 However, there is no universally accepted definition of a healthy or advantageous lung microbiome, in part because of our incomplete understanding of how the lung's resident microbiome and host immunity are related.
Lung microbiome and host metabolismIntestinal microbiome changes that deregulate the host metabolism have been thoroughly explored.42,43 According to reports, microbiome-derived carcinogens, including acetaldehyde and deoxycholic acid, are crucial in developing esophageal and liver cancers.44,45 Visconti et al. found metagenomic shotgun sequencing useful in the characterization of the gut microbiota while also analyzing the metabolites found in the blood and feces.46 Their results confirmed an association between gut bacterial metabolic pathways and metabolites in blood and feces.46 Through a variety of physiological processes, metabolism is crucial for preserving the homeostasis of the human body.47 Additionally, a recent study was conducted to explore the association between host metabolism and the lung microbiome. Weinberg et al. showed that lung microbiota can modulate the phenotypes of lung cancer tumor microenvironment components and can regulate the metabolic and inflammatory pathways.48 Given the importance of tumor microenvironment in cancer progression and treatment resistance,49 this finding can be applied in future studies to modulate the cancer biology. Cribbs et al. found that the glycerophospholipid pathways play a crucial role in the development of pneumonia in patients with AIDS. This study led to the identification of specific metabolic profiles associated with bacterial taxonomic resolution through sequencing the 16S gene in the bronchoalveolar lavage.50 In addition, Pseudomonas aeruginosa-based primary metabolites utilize substrates produced by other microbes, like Rothia mucilaginosa, which may take part in the etiology of cystic fibrosis.51 One of the potent metabolites is SCFA, which is produced in large amounts by microbiota in the large intestine which acts as a major signaling biomolecule in host cells. Even though various research investigations have focused on the role of SCFA in the host gut and immunity, its effects on the respiratory system, epithelium, and immune system remain largely unknown. S. aureus is one of the bacteria that animals are more likely to encounter, which may be influenced by the pulmonary Th17 immunity.52 Furthermore, several research findings have revealed that altering the gut microbiota in preclinical models may change the host immunity and delay response to infection.41
Microbiota and lung cancerRecent research has elucidated the complex interplay between microbial communities, tumor development, and immune cell response in the context of cancer. They elucidated the molecular pathways involved in oncogenesis and their potential significance. However, recent literature has revealed a robust bidirectional link between gut dysbiosis and lung oncogenesis. It is well-known that microorganisms can initiate tumor progression, mainly by generating bacterio-toxins and other pro-inflammatory factors.1,53 Due to the large surface area of the mucosal site and the constant connection with the outside environment, the lung is vulnerable to diverse microbes and environmental pollutants.
Lungs, for long, had been thought to be free from microbial contamination, but recent studies have revealed that they can be the home of a diverse commensal bacteria.54 The growth of such microbes is initiated through numerous factors, such as the presence of new bacteria, mechanical and immunological clearance, and the capability of microbes to reproduce under particular environmental conditions54 (Fig. 1). Interestingly, these microbial populations appeared to be reduced in diseased conditions such as cystic fibrosis, chronic obstructive pulmonary disease, and cancer.55,56 This finding clearly indicates an underlying complementary physiological role of these microbiotas to protect the lungs from infections and boost our immune system. To date, limited research has been done on the lung microbiota than on the gastrointestinal tract.
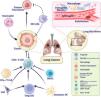
The function of the microbiome in lung cancer. Schematic illustration of lung microbiota to maintain homeostasis and cancer development. In the biology of lung cancer, the microbiota plays a number of functions and employs a range of techniques. The protagonists of the tumor microenvironment are bacteria found in the lungs and upper respiratory system, as well as those with intra-tumor and intracellular locations. The gut microbiota is crucial in influencing how the immune system reacts and how inflamed the body is. This is in addition to the bacteria involved in bloodstream translocation events. It's also possible that certain bacterial toxins with the potential to activate oncogenic pathways might alter lung function. Additionally, cigarette smoke and pollution are the two main causes of the dysbiotic changes in the lungs. Finally, the microbiome may affect metastatic pathways by increasing the production of vascular endothelial growth factors and promoting inflammation.
In a study conducted in non-smoking female patients with lung adenocarcinoma, a correlation was established between cancer stage and microbiota.53 In addition, two correlations (positive and negative) were established. A positive correlation was established between one set of microbiotas (Faecalibacterium as FB and T category) and primary tumor size. In contrast, a negative correlation was established between other microbiota (Fusicatenibacter saccharivorans and Bacteroides) and tumor size. Epidermal growth factor receptor (EGFR)-wild-type patients have abundant Bifidobacterium and FB, whereas Blautia was relatively low compared to the EGFR-mutated population.57 It is difficult to determine whether these changes had occurred before or after the development of cancer. It has been demonstrated that Bacteroides and Faecalibacterium can individually upregulate T-cells in the cancer lesion, followed by inhibiting proliferative cells and can activate Treg cells, respectively.58 Thus, these microbiotas may play a significant role in targeted cancer treatment as their biochemical metabolites may adversely affect the immune system. A few Gram-negative bacteria (Enterobacter, Haemophilus influenzae, and Escherichia coli) are capable of colonizing lung cancer. Patients with lung cancer showed low levels of Firmicutes and Proteobacteria compared to healthy individuals, whereas healthy individuals exhibited high numbers of Bacteroidetes and Firmicutes bacteria.59 These microbiotas persisted irrespective of how the bacteria in cancer evolved. Additionally, the gut microbiota and its metabolites trigger the toll-like receptor (TLR) response, which causes T lymphocytes to spread to distant areas when they pass through the epithelial barrier and enter the circulation.60
Studies have revealed a link between Enterococcus hirae and small cell lung cancer (SCLC) wherein bacteria were found to move from the gastrointestinal tract and enhance a tumor-specific response by activating TLR and subsequently triggered the formation of memory responses.53 There is growing proof that the causes of inflammation and bacterial infections may encourage the intestinal microbiota's transition from mutualism to a pro-carcinogenic form. Innumerable bacteria have been extensively investigated in terms of their molecular mechanisms, and researchers have explored their potential as a novel approach to cancer treatment.61 Within the realm of omics disciplines, proteomics plays a crucial role in the study of bacterial toxins. Advancements in proteomics have significantly improved the understanding of bacterial toxins, facilitating their characterization and contributing to the development of anti-cancer drugs based on these toxins.62 Bacterial endotoxins, such as LPS, are capable of entering the bloodstream through a leaky gut and may trigger systemic inflammation, potentially affecting the lung microenvironment. They may promote inflammatory responses that could potentially contribute to lung cancer development or progression.63 Altered gut microbiota composition has been linked to various diseases, including cancer. However, the specific gut microbiota profile in lung cancer patients has remained relatively unexplored. Zheng and colleagues conducted a study64 where they discovered a significant shift in the composition of gut microbiota in individuals with lung cancer compared to healthy individuals. In this study, the researchers analyzed the gut microbiota composition in a discovery group consisting of 42 early-stage lung cancer patients and 65 healthy individuals using 16S ribosomal RNA (rRNA) gene sequencing analysis. Bacteria can produce enzyme-active proteins that alter host cells’ DNA or interact with determining cell signaling pathways regulating inflammation, cell proliferation, and apoptosis.64 In prognostic studies, the lung microbiota's composition might be crucial. Identification and isolation of gut microbiota can be helpful for early-stage diagnosis of lung cancer.65Bacillus spp., found in lung secretions, has been associated with a higher risk of lung cancer,66 whereas the healthy group had greater levels of Bifidobacterium and Faecalibacterium. The microbiota in the sputum and stomach are dysbiotic. When considerable amounts of fiber are consumed, the concentration of short-chain fatty acids (SCFAs) increases in the blood, sputum, and stomach, indicating their entry into the lungs and airways.67 Overall, these findings demonstrate that microbiota is essential for developing lung cancer. Modifying the immune system and addressing the local and distal microbiota are components of a promising innovative strategy for the prevention and treatment of lung cancer.68
The role of microbiota in specific lung cancersImmune cells in the lungs help to maintain homeostasis, making the lung an important location of immunological–microbiota interaction. Lung cancer and bacterial dysbiosis are related, according to growing evidence from research on humans and laboratory animals.18 Lung cancer has been linked to decreased diversity, enrichment in particular genera of bacteria, and increased microbial colonies.69 Chronic lung infection may be the primary cause of cancer when microbial dysbiosis creates more hypoxemia, a micro-environment that augments cancer proliferation.70 The optional anaerobic characteristics of the bacteria that preferentially colonize tumors contribute to the observation that anaerobic respiration is increased in lung cancer. These microbiotas grow and worsen disease conditions by promoting and maintaining hypoxic conditions and establishing a pro-inflammatory tumor microenvironment.71 Notably, Jin and colleagues reported an exciting finding. They attempted to establish a link between the abundance of microbiota present in airways (rather than GIT microbiota) and the number of tumors developed in the lungs.37 They concluded that the local microbiota was substantially more important in the progression of lung carcinoma. Additionally, they showed that an intratracheally injected mixture of microbiota derived from developed lung malignancies significantly augmented tumor formation.37
There was no change in the beta diversity between the malignant lung tissues and healthy tissues. In contrast, patients with lung cancer have exhibited a reduction in alpha diversity of the microbiota tumor microenvironment as compared to the adjacent non-malignant lung tissues (Fig. 2).
Although there is no consensus on what makes for a healthy or sick lung microbiome, several researchers have discovered intriguing links between particular microbiota species (taxonomy or genera) and lung cancer. Jin and colleagues confirmed the significance of inter-immunity exchanges of the microbiota in supporting inflammation and the growth of lung cancer in a laboratory mice model.37 Tsay et al.72 discovered that, compared to controls, patients with lung carcinoma had higher levels of the oral bacterial population, mainly Streptococci and Veillonella. Tsay el al. found that PI3K and ERK (MAP kinase pathway) activation have been linked to the higher incidence of oral taxa. In this in vitro investigation, airway epithelial cells exposed to bacteria (Veillonella, Prevotella, and Streptococci) exhibited an upregulation of certain signaling pathways, such as ERK and PI3K pathways. Scientists attempted to link these signaling pathways to lung neoplasm as upregulation of these pathways by commensal microbiota dysbiosis promotes cancer development and progression.73 The presence of Staphylococcus in the microbiota is rare compared to Streptococcus, which is previously linked with lung cancer.74 This finding suggests that the former plays a harmful role and the latter a protective role in the growth of lung cancer. Another study, however, showed that while streptococcus may contribute to cancer prevention, Staphylococcus may cause DNA damage.75 Through specific microbial components like toxins, dysregulation of lung microbial populations probably fosters alterations to carcinogenic pathways.76 For instance, Apopa and colleagues77 found a significant amount of cyanobacteria in non-small cell lung cancer (NSCLC) samples and linked the cyanobacteria's toxin to inflammation-associated lung cancer growth. On the other hand, Yaghoobi and colleagues78 showed that some gram-negative bacteria's cytolethal distending toxin (CDT) have anticancer effects on A549 cell lines.
Incorporating a deeper mechanistic perspective into the review of probiotics’ potential in lung cancer management can significantly enhance its scientific rigor and utility for future research. To achieve this, the manuscripts should prioritize studies that offer comprehensive molecular insights. Key among these are investigations employing molecular biology techniques such as gene expression analysis and proteomics, which can elucidate the interactions between probiotics and lung cancer cells or the tumor microenvironment. An emphasis on signaling pathways is crucial, given that probiotics often exert their effects through pathways like NF-kB, PI3K/Akt, and MAPK, known for their roles in inflammation, cell proliferation, apoptosis, and immune responses.79 Additionally, the impact of microbial metabolites, such as short-chain fatty acids, on cellular processes offers a layer of mechanistic detail, highlighting how these substances modulate immune responses and exhibit anti-tumorigenic properties.80 The role of the immune system in mediating the anti-cancer effects of probiotics is another vital area of focus. Detailing the modulation of cytokine balance, activation of dendritic cells, natural killer cells, and T cells, and the potential induction of cancer cell apoptosis can provide a comprehensive overview of immune-mediated mechanisms.81–83
Probiotics have shown diverse impacts on the host and its immune system. Several bacterial strains have the ability to influence the environment within the gut, the protective barrier of the intestines, and they may control the immune system of the intestinal lining. Probiotics may influence several cells that play a crucial role in both the innate and acquired immune responses, such as dendritic cells (DCs), monocytes, Natural Killer (NK) cells, macrophages, lymphocytes, and epithelial cells. Specifically, they may stimulate the pattern recognition receptors (PRRs) found on both immune cells (such as M cells in Peyer's patches) and non-immune cells (such as intestinal epithelial cells). TLRs, among PRRs, have been extensively researched due to their ability to initiate signaling cascades that result in cell proliferation and the release of cytokines, thereby regulating the immune system. Research has shown that the immune system's response to probiotic bacteria may be attributed to the production of anti-inflammatory cytokines in the gastrointestinal tract. Particular strains of probiotics have the ability to stimulate dendritic cells (DCs), which then carry the antigens to nearby lymph nodes, resulting in the production of IL-10 and IL-12. In this context, Dendritic Cells (DCs) stimulate the development of unspecialized T and B cells into their specific subgroups, using a range of cytokines. Specifically, immature Th cells have the ability to develop into Treg, Th1, and Th2 immune cells. B cells may also transform into plasma cells, which are important in humoral responses, or regulatory B (Breg) cells, that contribute to the production of tumor growth factor (TGF)-β or IL-10. In addition, dendritic cells (DCs) may induce the activation of natural killer (NK) cells by producing cytokines including IL-12 and IL-15. Various Lactic Acid Bacteria (LAB) and other probiotic microbes may stimulate the production of IFN-γ by NK cells via the involvement of DCs.84
Based on the contribution of probiotics to intestinal health, it is currently believed that the core benefit of probiotics is to maintain healthy intestinal flora and support a healthy immune system through nonspecific and specific physiological effects (Fig. 3).85
The effects of probiotics on the host (SCFAs, short-chain fatty acids; sIgA, soluble IgA; GPR, G protein coupled free fatty acid receptor; DC cell, dendritic cell; Treg cell, regulatory T cell; Th17, T helper cell 17; ILC3, Type 3 innate lymphocyte; NK cell, Natural killer cell; LPS, lipopolysaccharide; TLR4, Toll-like receptor 4; NF-κβ, nuclear factor-κB).85
Probiotics, prebiotics, and synbiotics are examples of mature microbiome-targeting products that have just hit the commercial market and have demonstrated general safety in various clinical settings. The general effects include improved gastrointestinal steadiness, immunomodulation and obesity, and diet management by regulating microbial metabolism of dietary fibers by producing SCFAs, taking part in the digestive processes, and neutralizing inflammatory and carcinogenic agents, according to growing clinical evidence.86 On the other hand, pharmaceutical and biotechnological sectors are looking for microbial therapeutic techniques to develop and market chemotherapeutic and targeted cancer medicines. A clinical experiment revealed a therapeutic advantage of giving neomycin concurrently with irinotecan to minimize the adverse effects of chemotherapy.87 Chemotherapy-related side effects may be lessened by medications that target microbiomes. In different preclinical studies, small molecule inhibitors of bacterial glucuronidase were introduced to animals and were protected against diarrhea caused by anticancer drugs. However, the optimal ways to analyze the host microbiome are not well understood given the current state of research and information regarding the beneficial microbiota and molecular pathways.88 It is yet unclear if the microbial changes may result in unanticipated localized homeostatic abnormalities, inflammatory reactions, or even precancerous lesions. The function of existing probiotic strains can be enhanced by applying bioengineering techniques which involve the artifices of a gene of a probiotic strain for promoting the clemency to the technological stress, including but not specified to high temperature, oxygen, acidification, production process, and/or survival of the probiotic in the GIT, to offer significant function to the host.89 This advanced approach can be utilized to develop newer probiotic strains isolated from the pathogens. It permits for the development of proteins which were previously not present within the microorganism. Virulence factors of the pathogens can be reproduced and expressed into the probiotic strain and posterior administration of the attendant recombinant probiotic strain will inhibit the development of infection with no clinical expression of the signs. However, bio-engineered probiotics are able to transport therapeutics or vaccines, pathogens or toxins, promotes the immune response and imitate cell surface receptors.90 Bioengineered probiotics are currently being researched as to target transportation of definite genes identified toward a specific foodborne pathogen. The major limitations of bioengineered probiotics are they are categorized as genetically modified organisms (GMO).91
Probiotics in the management of lung cancerDistinct differences in the gut microbiota between lung cancer patients and healthy individuals have been documented, suggesting potential implications for prognosis and therapeutic strategies. By modifying the development of regulatory T cells, the gut microbiota may have a major impact on immunotherapy and may affect immunomodulation pathways.92 The same researchers discovered that using Akkermansia muciniphila supplements boosts immune therapy responsiveness, while aberrant gut microbiota composition is linked to treatment resistance.93 Immunotherapy, especially checkpoint inhibitors, has been widely applied in patients with lung cancer.94 However, a subset of patients experiences dramatic response to these agents. The predictive factors of response to checkpoint inhibitors are the subject of debate.95 Patients with lung cancer who react to immunotherapy have dramatically different gut flora from those who do not. Furthermore, it was demonstrated that in lung cancer patients, the predominance of the A. muciniphila species was positively associated with a considerably high response to anti-PD-1 therapy.92,93 It was further observed that a particular group of gut microbiota, namely Firmicutes, Proteobacteria, Actinobacteria, and Bacteroidetes, had boosted the response to anti-PD-1 immunotherapy.96 This effect might be due to the stimulatory effects of probiotics on the mitochondria of immune cells,97 which improves the immune cells’ activation following immunotherapy.98 Moreover, patients with NSCLCs who responded to nivolumab had a gut microbiota with a more varied makeup than those who did not. Additionally, it was discovered that patients with high microbiome diversity had longer progression-free survival time than patients with low microbiome diversity. A medical examination report of systemic immune disease assessed by flow cytometry showed that patients had a high concentration of CD4+ and CD8+ Tcells due to higher gut microbiome diversity, especially memory and natural killer (NK) cells in response to anti-PD-1.99 A retrospective study on 118 patients with advanced NSCLC receiving immunotherapy followed by supplement therapy with Clostridium butyricum (before and after immunotherapy) demonstrated a significantly longer progression-free survival and overall survival rate.100 Along with the known improvement in response to immunotherapy, these gut microbiotas can affect the lung cancer cells’ response to cytotoxic chemotherapies. In a lung cancer mouse model, for instance, oral administration of Lactobacillus acidophilus along with the anti-cancer drug cisplatin enhanced the anti-cancer efficacy of cisplatin by reducing the tumor size and increasing the survival rate. These findings suggest that the anti-neoplastic characteristics, such as pro-apoptotic and anti-growth activity of cisplatin, are promisingly moderated by introducing probiotics.101 Additionally, patients with advanced-stage lung cancer receiving E. hirae and Barnesiella intestinihominis together with chemotherapy and immunotherapy experienced prolonged progression-free survival.102 As a result, the improved immunomodulatory impact can be credited with enhanced survival of these individuals. However, further research is required to determine the involvement of the gut microbiota in the onset and lung cancer progression, as well as to study and assess the possible roles of the microbiome in the successful regulation of anticancer therapy.
Probiotic therapy and next-generation probioticsProbiotics, defined as specific bacterial strains that enhance human health without inducing antibiotic resistance in other microbiota members, have garnered attention for their therapeutic potential. Some of the bacteria in probiotics may travel to the colon to experience the specific gut ecology, metabolisms, and physiology. Probiotics may not always require settling in the targeted organs (gut and lungs).103 Probiotics, by definition must be safe for use in animals, tolerant to bile acids and acidity, and should be capable of adhering to and colonizing in the intestine. The information that is now available suggests that probiotic bacteria can have an impact on a range of immunological parameters, including humoral, cellular, and nonspecific immunity, as well as the immune system as a whole. New evidence suggests that probiotics affect nonspecific host defences and enhance NK cell function in aged individuals. In older mice receiving probiotic supplements, the age-associated decline in cytokine generation was shown to be reversed. Probiotics have resulted in enhanced immunomodulatory function by following several mechanisms such as influence of neutrophils and secretory IgA, induced development of mucus, activated macrophage by Lactobacilli signaling, inhibition of pro-inflammatory cytokine release, and by enhancing the peripheral immunoglobulin levels. Probiotics can also alter the cytokine secretion and phenotype of the DC surface. The management of toxicity, side effects, and inflammation brought on by chemotherapy in cancer patients appears to require other microorganisms. The administration of gemcitabine along with probiotics containing specific microbiota such as Lactobacilli and Bifidobacteri, has demonstrated noteworthy efficacy in the reduction of hematological toxic impact, influencing RBCs, platelet count, as well as improved aspartate aminotransferase (AST) level, gamma-glutamyl transferase (GGT), creatinine, and urea levels in mice as compared to mice receiving chemotherapy alone.104 The vast majority of regularly prescribed traditional probiotics, such as Saccharomyces species, Enterococcus, Streptococcus Bifidobacterium, Lactobacillus, and numerous others, were selected by chance or as a result of a personal experience of an individual. Traditional probiotic administration, on the other hand, does not specifically target any disorders. Many prospective NGPs are now being developed vigorously thanks to investigations employing the most recent generation of sequencing and bioinformatics tools. A class of organisms created specifically for medicinal purposes and as novel preventive interventions is included in emerging NGPs.
Probiotics in the management of lung cancerIt is required to comprehend how the lung microbiota is associated with the wellness and disease of the lungs, including lung metastasis. Therefore, to accomplish the right diagnosis and treatment of lung carcinogenesis, discovering new therapeutic target(s) for the lungs’ microbial environment is essential.105 Identifying whether the microbe has a direct role in disease pathogenesis or whether the healthy microbial population of the person declines after the onset of the disease is crucial. Though there is still a paucity of evidence supporting the prevention of lung cancer with probiotics, certain research has found promising results. According to a study, a healthy intestinal microbiota may act as a preventative measure in the treatment of cancer.106 It has been suggested in a newly reviewed body of literature that metagenomics, meta-transcriptomics, and culturomics platforms are making it possible to compare the cancer patients’ and healthy subjects’ microbiomes. Together, the data may indicate which bacterial genera or species would be helpful to patients.107 Therefore, using microorganisms or their by-products to treat cancer may also be able to treat tumors. It was found that the development of cancer-initiating toxins and metabolites by bacteria may also have a crucial role on cancer progression. In order to directly target the malignant cells in future treatments, it will be necessary to combine the use of immunotherapeutics and more traditional methods with the use of microbiomes and their products.
Radiotherapy and chemotherapy are the mainstay of cancer treatments.108,109 The application of chemotherapy and radiotherapy is limited by their severe adverse effects.110 Recently, it has been demonstrated that probiotics can be applied in reducing the adverse effects of radiotherapy and chemotherapy.111Table 1 summarizes the studies examining the use of probiotics in the treatment of people with the progression of lung carcinoma, animals bearing lung cancer, and a few in vitro research studies employing lung and other cancerous cell lines. These studies documented how probiotics affect lung metastasis, checkpoint inhibitors, homeostasis, and the increased effectiveness of anti-tumor drugs. Lung cancer treatment with recombinant probiotic bacteria, particularly Bifidobacterium infantis, have also been discussed.5,101,102,104,106,112–119
Impact of probiotics, applied models (in vitro and in vivo), and major findings in the treatment of lungs tumor and metastasis.
| Cells/disease | Study type | Probiotic strain/animal model | Findings | Ref. |
|---|---|---|---|---|
| Lewis lung carcinoma (LLC) (3 lines) and hepatoma (10lines) | In vivo | Lactobacillus casei in mice and guinea pig animal models | Increased longevity and prevention of pulmonary metastases. | 104 |
| Solid tumor (sarcoma 37) and metastatic 3LL | In vivo | Saccharomyces cerevisiae 14K and Enterococcus faecium K-50 (C57B16 mice and Balb/c) | Prebiotic significantly decreased metastases compared to animals that just received the vaccination. | 112 |
| Lung cancer | In vivo | Gene (sFlt-1 gene) transfer system through Bifidobacterium infantis. A recombinant technology in mice | LLC C57BL/6 mice with reduced tumor development and longer survival times. | 113 |
| Lung cancer | In vivo | Bacterium (Bifidobacterium infantis) mediated receptor (sKDR) insert in mice. sKDR is a soluble kinase insert domain receptor | By boosting the tumor's rate of necrosis and extending the mice's survival, the tumor grows less quickly (LLC C57BL/6 mice). | 106 |
| Lung metastasis of melanoma | In vivo | Commensal microbiota (C57BL/6 mice) | Following antibiotic therapy, changes in the microbiota led to the activation of NK cells. | 114 |
| Lung cancer | In vivo | Lactobacillus acidophilus (C57BL/6J mice) | Cisplatin's anti-tumor effects were enhanced when combined with L. acidophilus and diminished when combined with ABX. Probiotics and cisplatin given together boosted survival rates. | 101 |
| Breast and lungs cancer | In vitro | Lactococcus lactis NK34 | >77% of cytotoxic activity. | 5 |
| Lung, breast, and cervical cancer | In vitro | Lactococcus lactis KC24 | Strong cytotoxic action, excluding cells that are cervical cancer-related. | 115 |
| Melanoma | In vivo | Bifidobacterium breve, B. cocktail, B. lomgum, B. bifidum, and B. lactis in C57BL/6 mice | The similar level of tumor control as PD-L1-specific antibody therapy (checkpoint blockade). | 116 |
| Metastatic melanoma or non–small cell lung carcinoma (NSCLC) | In vivo | Germ free mice and Bacteroides fragilis | Immunostimulatory effects of CTLA-4 blockade. | 117 |
| Breast cancer and lung metastasis | In vivo | Lactobacillus. casei and CRL 431 BALB/c mice | Reduced tumor vascularity, extravasation of tumor cells, and lung metastasis; decreased tumor growth. | 118 |
| Breast cancer and lung metastasis | In vivo | Kefir used as a probiotic with fermented milk/BALB/c mice | Significantly less metastases to the lung and bone marrow and an increase in helper and cytotoxic T cells. | 119 |
| Advanced lung and ovarian cancer patients | In vivo | Barnesiella intestinihominis and Enterococcus hirae/C57BL/6J mice | Enhanced antitumor properties of cyclophosphamide. | 102 |
Table 2 summarizes the potential therapeutic impact of diverse probiotic strains on the prevention of various cancer types. Concurrently, the table discusses the plausible anticancer effects associated with these probiotics, providing a comprehensive overview of their preventive capabilities.100,120–132
The role of probiotics in different cancer types and the involved mechanisms.
| No | Anticancer drugs | Cancer type | Antibiotic mechanism | Ref. |
|---|---|---|---|---|
| 1. | Bifidobacterium lactis Bb12, Lactobacillus rhamnosus GG, Lactobacillus rhamnosus 573Bifidobacterium longum, Lactobacillus acidophilus, Enterococcus faecalisLactobacillus acidophilus LA-11, Lactobacillus acidophilus | Colorectal cancer | ↓ Chronic inflammation associated with cancer↓ WT1-expressing tumor growth↓ Azoreductase, nitroreductase and glucuronidase activity | 120,121122123,124 |
| 2. | Propionibacterium, freudenreichii subsp. Shermanii | Liver cancer | ↓ Significantly in concentration of urinary AFB-N7-guanine | 125 |
| 3. | Lactobacillus reuteri PTCC 1655, Lactobacillus kefiri P-IF | Gastric cancer | Inhibits cell proliferation↑ Apoptosis | 126127 |
| 4. | Lactobacillus acidophilus, Bifidobacterium bifidum | Cervical cancer | ↓ Incidence of radiation-induced diarrhea | 128 |
| 5. | Clostridium butyricum MIYAIRI 588 | Non-small cell lung cancer | ↓ Intestinal epithelial damage, Improve immune checkpoint blockade | 100,129 |
| 6. | Enterococcus faecium 12a | Cervical cancer, lung cancer | Inhibit proliferation of cancer cells | 130 |
| 7. | Enterococcus thailandicus | Liver cancer | Inhibition of hepatocellular carcinoma cell growth | 131 |
| 8. | Enterococcus mundtii C4L10 | Breast cancer, lung cancer and colon cancer | Apoptogenic, antimicrobial, and antiproliferative properties | 132 |
The potential role of probiotics in supportive care or symptom management for lung cancer patients has gained increasing attention in recent years. Probiotics, defined as live microorganisms that, when administered in adequate amounts, confer a health benefit on the host, have been explored for their ability to alleviate treatment-related side effects and improve the overall quality of life in cancer patients, including those undergoing treatment for lung cancer.
Research indicates that probiotics may play a significant role in managing gastrointestinal side effects commonly associated with cancer treatments such as chemotherapy and radiation therapy. These side effects often include diarrhea, constipation, and mucositis, which can severely impact patients’ quality of life. Probiotics are thought to restore the gut microbiota balance disrupted by cancer treatments, thereby reducing the incidence and severity of these gastrointestinal symptoms. For example, a study found that probiotic supplementation reduced the severity and duration of radiation-induced diarrhea in patients with abdominal and pelvic cancers, including a subset of lung cancer patients undergoing combined chemoradiation therapy.124 Similarly, another clinical trial demonstrated that probiotics could effectively prevent chemotherapy-induced diarrhea in cancer patients, suggesting their potential role in supportive care across various cancer types, including lung cancer.133
Beyond gastrointestinal symptoms, probiotics have been investigated for their potential to enhance overall immune function, which could be compromised in lung cancer patients undergoing treatment. A robust immune system is crucial for managing infections and maintaining general health, which, in turn, can improve patients’ quality of life. Research has shown that probiotic intake can increase the activity of natural killer cells and other aspects of the immune response in elderly patients, which could be beneficial for lung cancer patients experiencing immunosuppression due to their treatment or the cancer itself.134
Furthermore, there's a growing interest in the psychological benefits of probiotics, given the gut–brain axis's role in regulating mood and cognitive functions. Given that lung cancer patients often experience high levels of stress, anxiety, and depression, probiotics could offer a supportive care avenue to improve mental health and quality of life. A recent review had highlighted the potential of probiotics in reducing symptoms of depression and anxiety, which could be particularly relevant for patients coping with the psychological burden of cancer.135 Overall, the exploration of probiotics as a supportive care option in lung cancer treatment is promising. Their potential to mitigate treatment-related side effects, enhance immune function, and possibly improve psychological well-being could significantly contribute to the holistic management of lung cancer patients, improving their quality of life during and after treatment. However, more targeted clinical trials and research are needed to fully understand the scope and mechanisms of probiotics in this context and to develop specific, evidence-based recommendations for their use in lung cancer patients.
Practical considerations for incorporating probiotics in lung cancer treatment protocolsIntegrating probiotics into lung cancer treatment protocols presents a promising research direction with the potential for tangible clinical benefits, yet necessitating careful consideration of several practical aspects to ensure both feasibility and safety. The selection of specific probiotic strains is paramount, as their effects can vary significantly. Strains such as Lactobacillus rhamnosus GG and Bifidobacterium longum are notable for their demonstrated benefits in lung health and cancer mitigation through preclinical studies, emphasizing the importance of strain specificity.136,137 Additionally, the quality and viability of these strains are critical for their effectiveness, necessitating recommendations for products adhering to rigorous manufacturing standards to ensure the presence of live, active cultures.138 However, the optimal dosing and timing for administering probiotics within lung cancer therapy remain undefined, with ongoing research aiming to establish these parameters to enhance the therapeutic benefits while minimizing potential interactions with conventional treatments.139 The potential challenges in incorporating probiotics into cancer care include interactions with existing cancer therapies, such as chemotherapy and immunotherapy, which require a deep understanding to prevent adverse effects and safeguard patient safety.120 Furthermore, the immunomodulatory properties of probiotics call for caution in patients with compromised immune systems, due to risks like bacteraemia or fungemia.140 Another significant hurdle is the variability in probiotic formulations and the regulatory landscape across different jurisdictions, underscoring the need for standardized formulations and guidelines to facilitate clinical application.141 Conclusively, while the use of probiotics in lung cancer treatment schemes opens new avenues for improving patient outcomes by exploiting the gut–lung axis, the realization of their full potential hinges on the outcomes of rigorous clinical trials aimed at determining their efficacy, safety, and optimal use in this particular cohort. The successful integration of probiotics into clinical practice will likely depend on a collaborative effort among researchers, healthcare practitioners, and regulatory authorities to overcome the intricate challenges associated with their implementation in cancer care.
Key knowledge gaps and future research directionsAddressing the role of probiotics in lung cancer management, especially in terms of supportive care and symptom alleviation, necessitates a critical understanding of the limitations inherent in the current body of research, alongside a keen identification of existing knowledge gaps. These gaps not only underscore our present understanding but also chart a course for imminent research endeavors. By delineating these areas explicitly, we will be positioned to articulate specific research questions and hypotheses that could catalyze future investigative efforts, thereby enhancing our grasp and utilization of probiotics in the context of lung cancer treatment.
Among the pivotal areas awaiting exploration is the identification of optimal probiotic strains and combinations that are particularly efficacious for lung cancer patients. Despite various strains being acknowledged for their health-promoting attributes, discerning those that specifically ameliorate treatment-related side effects and bolster patient quality of life are imperative. Comparative analyses across different strains could shed light on benefits uniquely pertinent to lung cancer patient care.142 Furthermore, the establishment of effective dosing regimens and appropriate timing for probiotic administration within lung cancer therapeutic protocols remains elusive. There's a compelling need for dosing studies to devise evidence-backed guidelines that amplify probiotics’ therapeutic impacts, with investigations into the timing of administration potentially optimizing efficacy and mitigating adverse reactions.143
The interplay between probiotics and conventional lung cancer therapies, such as chemotherapy and radiation, presents a fertile ground for research into possible synergistic effects that might amplify treatment outcomes or diminish negative side effects. The dynamics between probiotics and emerging therapies, including immunotherapy, also warrant thorough exploration.144 Additionally, while the advantageous effects of probiotics are increasingly acknowledged, the mechanisms underpinning these benefits, especially within the cancer treatment milieu, demand deeper investigation. Studies at the molecular and cellular levels could illuminate the interactions between probiotics, the host immune system, the microbiota, and cancer cells, providing valuable mechanistic insights.145
The call for more rigorous clinical trials is unmistakable, with an emphasis on not just evaluating the safety and efficacy of probiotics for lung cancer patients but also on patient-centered outcomes such as quality of life and treatment satisfaction. These investigations should account for patient diversity and the influence of variables like age, sex, cancer stage, and treatment modality on the effectiveness of probiotics.146 Lastly, the long-term implications of probiotic use on lung cancer survivorship, including its potential in bolstering immune health, preventing recurrence, and enhancing overall well-being, represent a significant domain for future research.147 This comprehensive approach to investigating probiotics in lung cancer management underscores a multidimensional endeavor that could significantly advance our understanding and application in this field.
ConclusionsEmploying biotherapeutics for the treatment of respiratory diseases, including lung cancer, constitutes a forward-thinking approach. These microorganisms primarily alter the gut microbiota through immunomodulation. As we learn more about the relationship between micro-organisms in various organ systems and the immune system, it will be easier to design probiotic-based therapies. Even though clinical trials have produced intriguing and good results, more research is needed to identify optimal strains, precisely describe reaction apparatuses, and assess the effects of probiotics to fully realize the benefits. Specific forms and stages of lung cancer call for extensive human clinical trials. In our opinion, various probiotic bacterial strains, in combination with additional immunotherapeutic medications or different forms of therapy, may be accepted as further therapy for the prevention or management of lung cancer.
Authors’ contributionsBlinded for review.
Institutional review board statementN.A.
Informed consent statementN.A.
Data availability statementN.A.
FundingN.A.
Conflicts of interestThe authors declare that they have no competing interests.
Sourav Mohanto would like to acknowledge Yenepoya (Deemed to be University), India for providing the research facilities and resources to draft the manuscript.